Global dossier management is a hot topic for many small and large organizations in life sciences.
Global dossier management is a challenging concept on its own; and people involved should be aware of multiple aspects.
In our LinkedIn Live Session with Steve Gens (Managing Partner at Gens & Associates) and Preeya Beczek (Regulatory Affairs Expert at Beczek Consulting) and Paul Ireland (VP Life Sciences at DocShifter) we answered the frequently asked questions around global dossier management.
In the video recording, you will find answers to these questions, and many more:
- How and where is industry investing in dossier management? What is the business case?
- What plays a major role in global dossier management?
- What are the evolving roles and common challenges for global dossier management for small and large organizations?
- How is technology evolving to support dossier management?
- Do dossier managers realize any benefits from structured content management?
- How do companies implement submission management as a role, where there may not be one today?
Enjoy!
Visuals from the Session
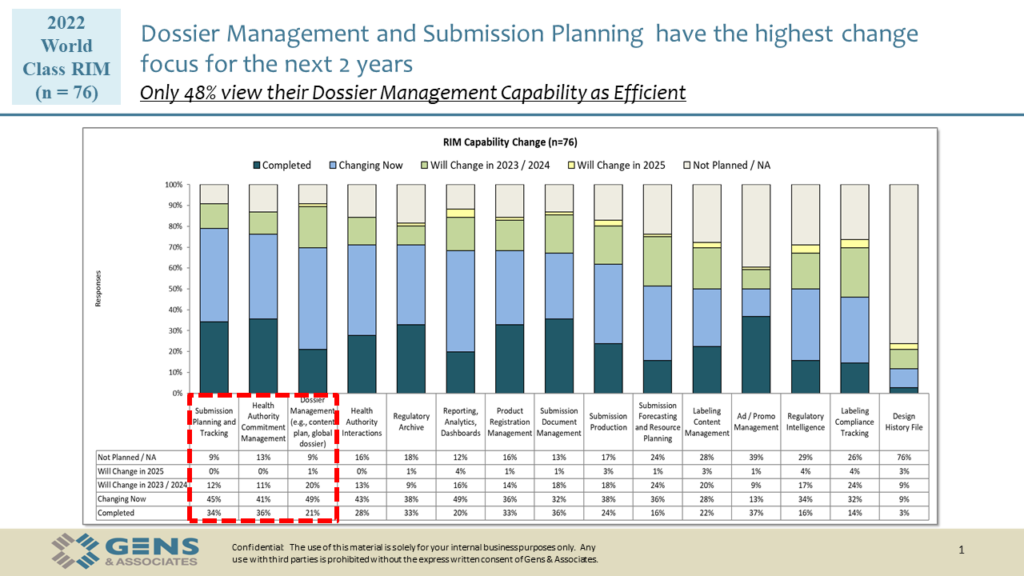
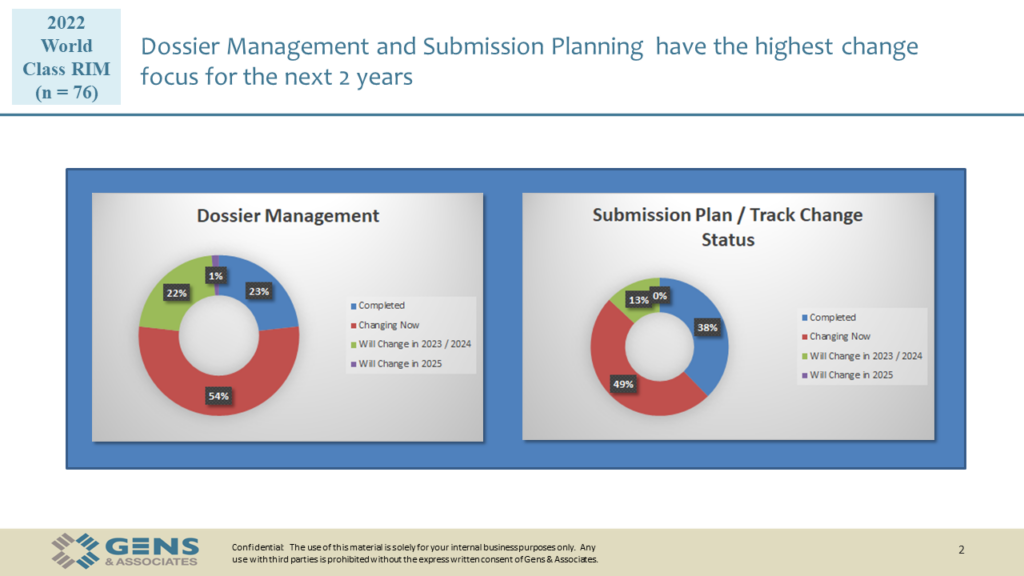
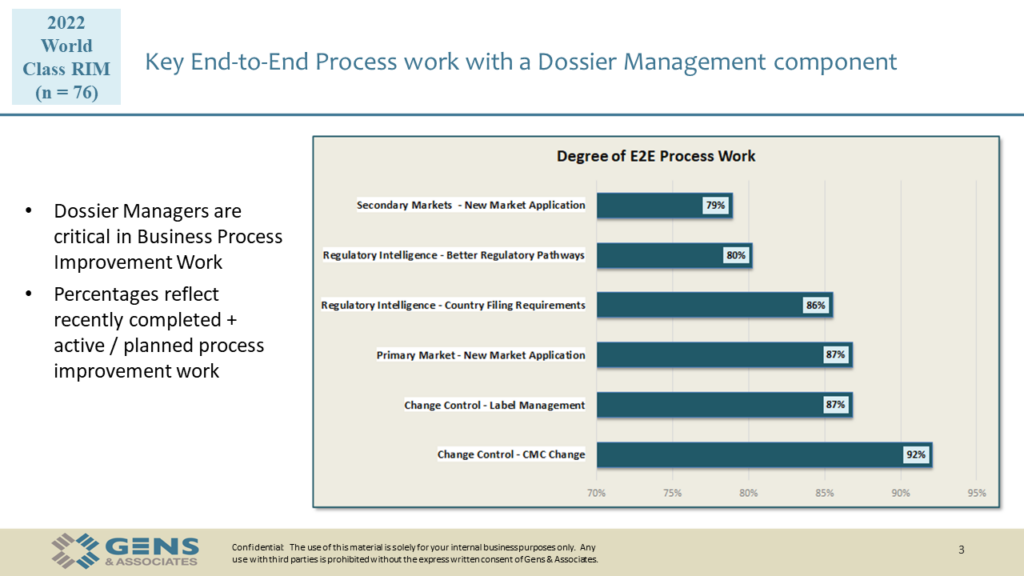
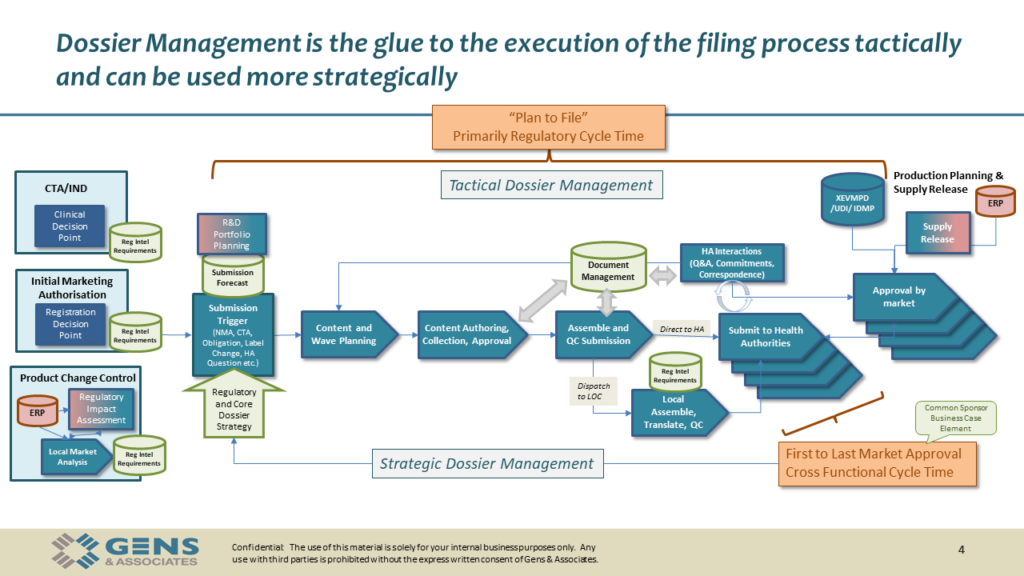
Here are the visuals we’ve used during the session, available for download.
Interested in finding out more about the 2022 World Class Regulatory Information Management (RIM) Study Whitepaper? You can download it here.
Keywords: Global dossier management, regulatory submissions, Steve Gens, Preeya Beczek, pharmaceuticals, drug manufacturing, life sciences




