Accelerating regulatory document preparation in Life Sciences
Automate PDF conversion, enrichment and quality control.
- Scalable, compliant and fast
- On premise, or in your cloud
- Integrates seamlessly with all your existing systems
Trusted by top Pharma companies globally



















Built together with you: streamlining complex document conversion
Our mission is to accelerate document preparation in highly regulated industries. We do this through automation and advanced file format conversion & enrichment. For 20 years now, we’ve helped our customers automate complex document conversion flows, save tremendous amounts of time and money and accelerate regulatory approvals.
We stay close to our customers, continuously adapting to their needs and exceeding expectations. And as we continue to innovate, this journey is far from over — stay tuned for what’s next!

Eliminate manual document preparation and accelerate regulatory approvals
Life sciences organizations often struggle with time-consuming, error-prone document conversion flows that delay regulatory approvals and increase costs. DocShifter solves these challenges by automating document conversion, eliminating manual effort, and ensuring compliance. This reduces submission times, minimizes errors, and frees up valuable resources, allowing teams to focus on higher-priority tasks and accelerate time to market.
60%+ Time Savings
Automates document prep. and PDF conversion. Result? Saving costs, reducing manual effort and speeding up submissions.
10x Reduction in Conversion Failures
Ensures smooth workflows with fewer errors, critical for regulatory documents.
High-Volume Document Handling
Supports large-scale implementations with minimal errors, boosting operational efficiency.
Compliant,
Submission-ready PDFs
Fully automated submission-ready PDF prep. that meets global standards (FDA, EMA, PMDA), accelerating time-to-market.
Want to see DocShifter in action?
Explore how Life Sciences companies use DocShifter
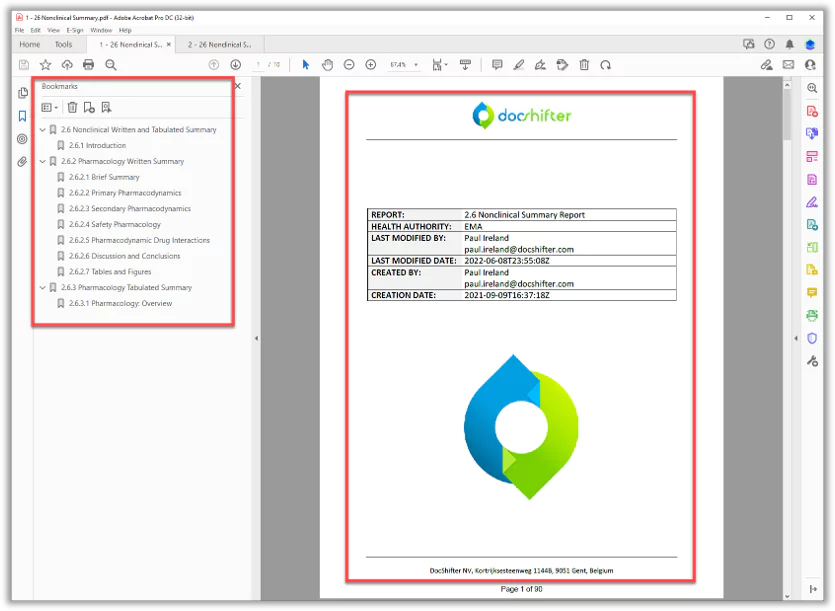
Submission Ready PDF
Compliant, submission ready PDFs for multiple health authorities. Without any manual work.
Automated Report Level Publishing
Automated report-level publishing for truly searchable PDF reports. Without the headache.
Digital Document Archiving
Future-proof your digital document archiving with powerful file format conversion.
Incoming Email to PDF Conversion
Monitor email inboxes and streamline email to PDF conversion.
Validate and fix Word files for submissions
Automatically check & fix Microsoft Word documents for styling and formatting errors.
Validate and fix PDF files for submissions
Check and fix your PDFs to make sure they meet internal and external guidelines.
Image, Audio & Video Conversion
Automatically convert images, audio & video files to the format of your choice.
Fully Integrated with Veeva, LORENZ, SharePoint and more...
Documents live in different platforms; shapes or forms. DocShifter acts as the centrepiece for all your conversion & enrichment needs.
How? By seamlessly connecting with all your existing systems: Veeva, LORENZ, OpenText Documentum, Ennov, Generis CARA, Microsoft SharePoint, file shares, email repositories, API & web services.
The result? Standardized, consistent, high quality documents. No matter which system they come from or go to.
*All third-party company names and logos are trademarks™ or registered® trademarks of their respective holders. Use of them does not imply any affiliation with or endorsement by them.
Partnering with confidence
DocShifter streamlines submissions for a large US-based biotech company by generating 30,000 compliant ready PDFs every month.

A US-based biotech company achieves 60% time savings in document preparaiton and speeds up time to market by 30% thanks to automated PDF checking and fixing.

No more manually merging Microsoft Word files into PDF reports. Automated Report Level Publishing for 510k and PMA Submissions for a Medical Devices Company.


PharmaLex, a technology-enabled solution provider in the Life Sciences industry, partnered with DocShifter to streamline their complex PDF-submission process for their 1000+ clients worldwide. So what changed?

Support you can count on. No bots, just experts.
At DocShifter, we don’t just talk the talk — we walk the walk when it comes to customer service. Expect real people in every interaction, not robots, and a two-hour response time for any support you need.
Whether it’s via live chat or phone, our expert team is always ready to help with your regulatory document conversion needs. Fast, reliable, and personal — this is support like no other.
Discover why companies of all sizes trust us for their document conversion
Fully automated
Convert any file type without the need for manual intervention. Simply set up and start converting.
Securely install anywhere
Because security and privacy matter, convert your documents on-premise or in your cloud. On Windows or Docker.
The best customer service in the industry
With our support team guaranteeing two-hour response times, help is always at hand.
Superior conversion speed
Without the need for MS Office or Adobe, DocShifter converts documents 10x faster than comparable solutions.
Seamless integration
Effortlessly integrate all of your enterprise systems, both natively or via web API.
High-availability
With zero downtime, your conversion service will always be on to meet the demands of your business.
Looking to automate your document conversion?
Get in touch with us.
Frequently Asked Questions
High volume, high-quality document conversion, on-premise or in the cloud.
Automation, compliance, quality, speed, scalability, and configurability is why regulated enterprises choose DocShifter. In Life Sciences, we support the regulatory teams by:
- Accelerating the drug submission process through automation of renditions for submission-ready PDF documents in eCTD submissions.
- Automating checks and fixes to Word and PDF files to ensure that pharma companies meet the most stringent technical requirements by the health authorities (FDA, EMA, PMDA, Health Canada).
- Automatically merge documents into a single PDF and generate (compliant) reports for submission and documentation. Including 510k and PMA medical device submissions.
- Convert all required digital files for storage in a long-term archive. PDF/A or the latest file format in use to ensure digital sustainability.
For MS Office, image, audio and video files. In Banking & Insurance & Government, we support IT staff to:
- Convert millions of documents to the desired file format, such as PDF or PDF/A. Or to the latest version (f.e. .xls to .xlsx)
- Standardize the file format used in customer communication or in a digital mailroom.
- Convert document to a future-proof file format for archiving. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce Risk. Reduce IT infrastructure costs.
DocShifter has native integration with the most desired document management/regulatory information management systems: Ennov, Generis CARA, OpenText Documentum, Microsoft SharePoint, Veeva Vault.
DocShifter can also be set up to monitor file shares, local drives, email inboxes. API service functionality is also available to make file format conversion available in any system.
Folder monitoring in document conversion refers to the process of continuously observing or scanning a designated folder or directory for any changes or additions to its contents. This is often used in the context of document management and conversion systems.
Here's how it typically works:
Selection of a Monitored Folder: A specific folder or directory on a computer or network is chosen for monitoring.
Continuous Scanning: The system constantly checks this folder for any new files, modifications, or deletions.
Document Conversion: When a new document is added or an existing one is modified, the system automatically initiates a document conversion process. This process may involve converting files from one format to another (e.g., PDF to Word or vice versa), extracting information, or performing other relevant transformations.
Automated Workflows: Folder monitoring is often part of an automated workflow system. Once a change is detected, predefined actions or workflows are triggered without requiring manual intervention.
Real-time Updates: The monitoring is usually done in real-time or with short intervals, ensuring that the system is responsive to changes as soon as they occur.
DocShifter is extremely easy to set up, configure and use.
The drag-drop user interface, and the ability to use it on any browser, makes it extremely user friendly.
To have an overview of what the training provided, please visit our Training section.




