A closer look into the eCTD triangle and its modules
-
By Othmane Achir
- 3 minutes read
What is the eCTD (Electronic common technical document) ?
It is used for the preparation of applications for new drugs that need to be submitted to the regional regulatory authorities in all participating countries across Europe, Japan and the United States.
The paper CTD is destined to be replaced by the electronic version, called eCTD, and makes regulatory review processes easier.
What are the modules of CTD (common technical document)?
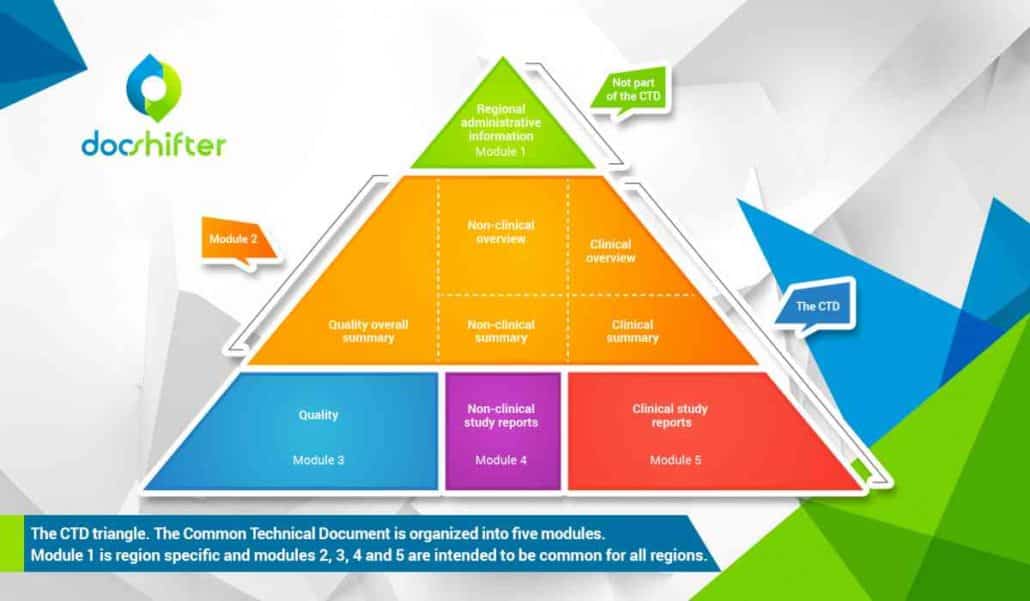
The CTD is organized into five modules. Module 1 is region specific and modules 2, 3, 4 and 5 are intended to be common for all regions.
Module 1
Module 1 is not part of the CTD and contains administrative information and prescribing information that is region specific. These documents can be forms, cover letters, labels, brochures, etc.
Module 2
Module 2 consists of the CTD summaries and starts with an introduction to the specific drug, its pharmacological class, mode of action and proposed clinical use. It should also provide an overall summary of the quality information that is provided, the (non)-clinical overview, as well as the (non)-clinical summary
Module 3
Module 3 is the quality of the pharmaceutical documentation. For example, everything from the drug substance information (name, manufacturer), to the drug product information (name, dosage form, formula, …).
Module 4
Module 4 consists of the non-clinical study reports like pharmacology and toxicology.
Module 5
Module 5 contains clinical study reports, like clinical trials.
How can DocShifter help you with your eCTD submissions?
Are you a pharmaceutical or biotech company and are you interested in drastically improving your eCTD publishing solutions ?
DocShifter’s compliant submission ready PDF solution creates fully compliant submission ready documents from your source documents: Microsoft Word, Excel, PowerPoint, scanned PDFs, images, text files, HTML and XML files and many more. For any health authority in the world: FDA, EMA, PMDA, HC, SwissHealth and many more.
These documents are ready to be fed into any publishing solution you are using, while allowing QC steps to be performed earlier in the process.
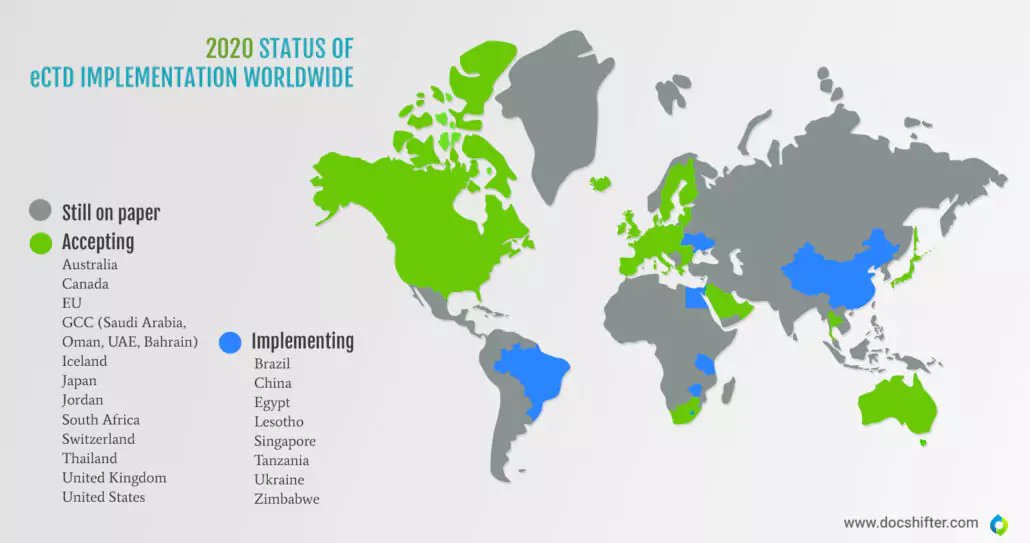
Did you know this? Many countries still submit on paper.
Did you enjoy this blog post? We have more free knowledge-articles available for you.
Thank you very much for reading our blog post, we hope you enjoyed it.
For more free articles on regulatory topics (eCTD, regulatory submissions, RIM, eCTD v4.0, etc.) we invite you to join our free LinkedIn Regulatory Community.
This community is where we share tips & tricks, updates and learnings on regulatory topics. The community currently has 2500 members and is growing quickly.
-
The PDF format specifications checklist for FDA submissions.
- eCTD 4.0: Key Changes & Impact on Submission Content Preparation
If you think this is interesting, I would like to personally invite you to join the Regulatory Professionals Community on LinkedIn. And don’t worry, it is completely free.
About DocShifter
Speed, quality, scalability, and configurability are reasons why Life Sciences organizations choose DocShifter to generate technically compliant, submission-ready PDF. High volume, high-quality document conversion, on-premises, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce risk. Reduce IT infrastructure costs.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.
Last update: 08/01/2021