How to automate PDF submission content preparation to speed up publishing
-
By DocShifter
- 4 minutes read
1. Save hundreds of hours every month when preparing PDFs for submission-readiness
If regulatory operations teams could have a wish, one would be to have all documents formatted properly, according to the guidelines.
Unfortunately, even when external and internal resources work with perfectly-styled Microsoft Word templates, it is no surprise that almost everyone has their way of working with those.
Some people work on Mac, Windows, PC, tablet, etc. where the template isn’t always followed. Everyone works with different macro’s. And regulatory operations are responsible for formatting documents, no matter whatever condition they are in, to become submission-ready.
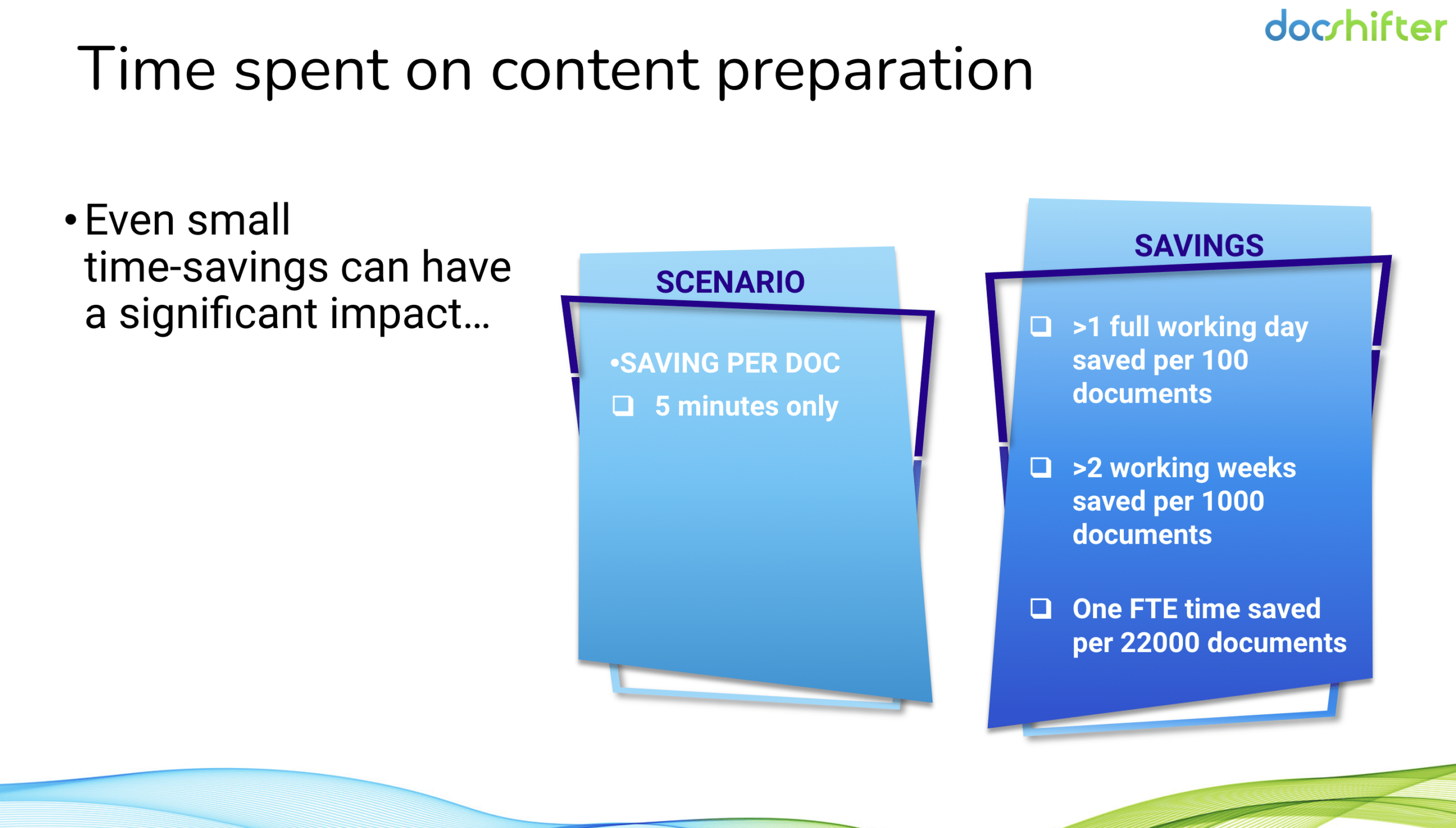
What if there was a way to save 100s of hours every month when preparing PDFs? Not talking about formatting only, but also looking for issues in the PDFs take a lot of time.
In a scenario where regulatory operations spend 20 minutes on each document, even a time-saving of 5 minutes per document makes a huge impact.
How? Through automation.
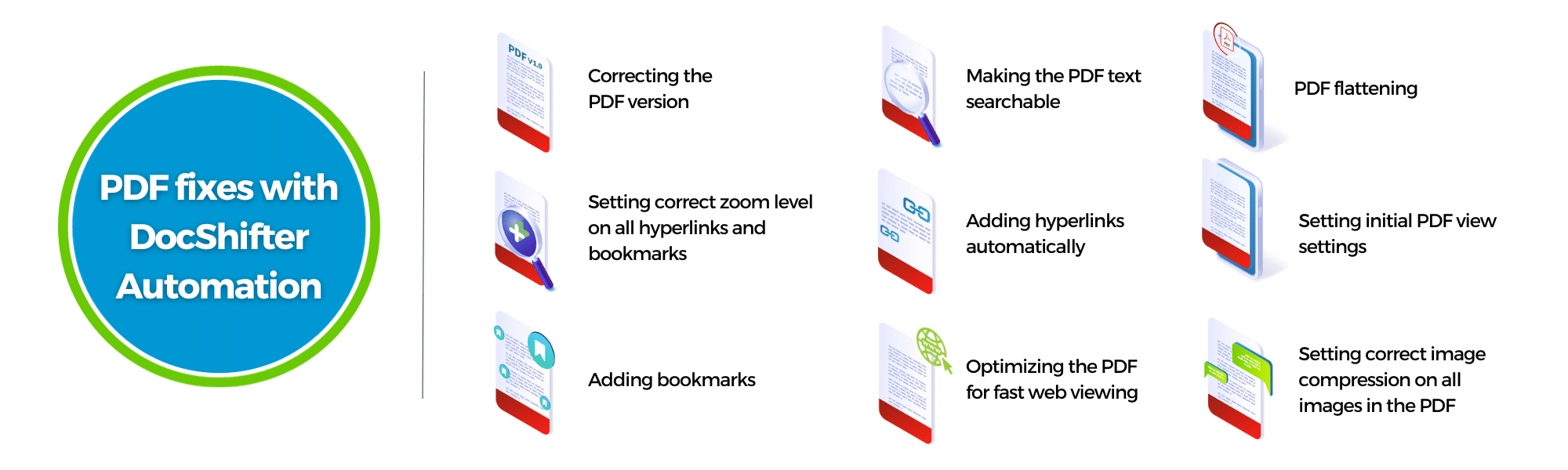
DocShifter, next to formatting your PDF files completely automatically to the FDA, EMA, PMDA specifications; is also able to tell you where most common errors are in your PDF files.
Faulty bookmarks, hyperlinks that do not contain any links, wrong text color, fonts used that are not allowed, font size, table of contents, and many others. Things that you spend time on today, document per document, can be fully automated.
2. Know exactly where to look for in your PDF files for non-compliance elements
It was one of our customers that opened our eyes. We spend a lot of energy talking about how good we are at converting documents to PDF’. Until a customer told us before the webinar that PDF conversion is the easy part. And not the problem.
DocShifter enables the identification and resolution of issues in Microsoft Word and PDF documents.
DocShifter can be configured to perform checks for non-compliance issues related to comments, paragraphs, section numbering discrepancies, bookmark and tables, fonts, hyperlink, image-related concerns, font size, text that is blue but isn’t a hyperlink, fast web view optimisation, and many more.
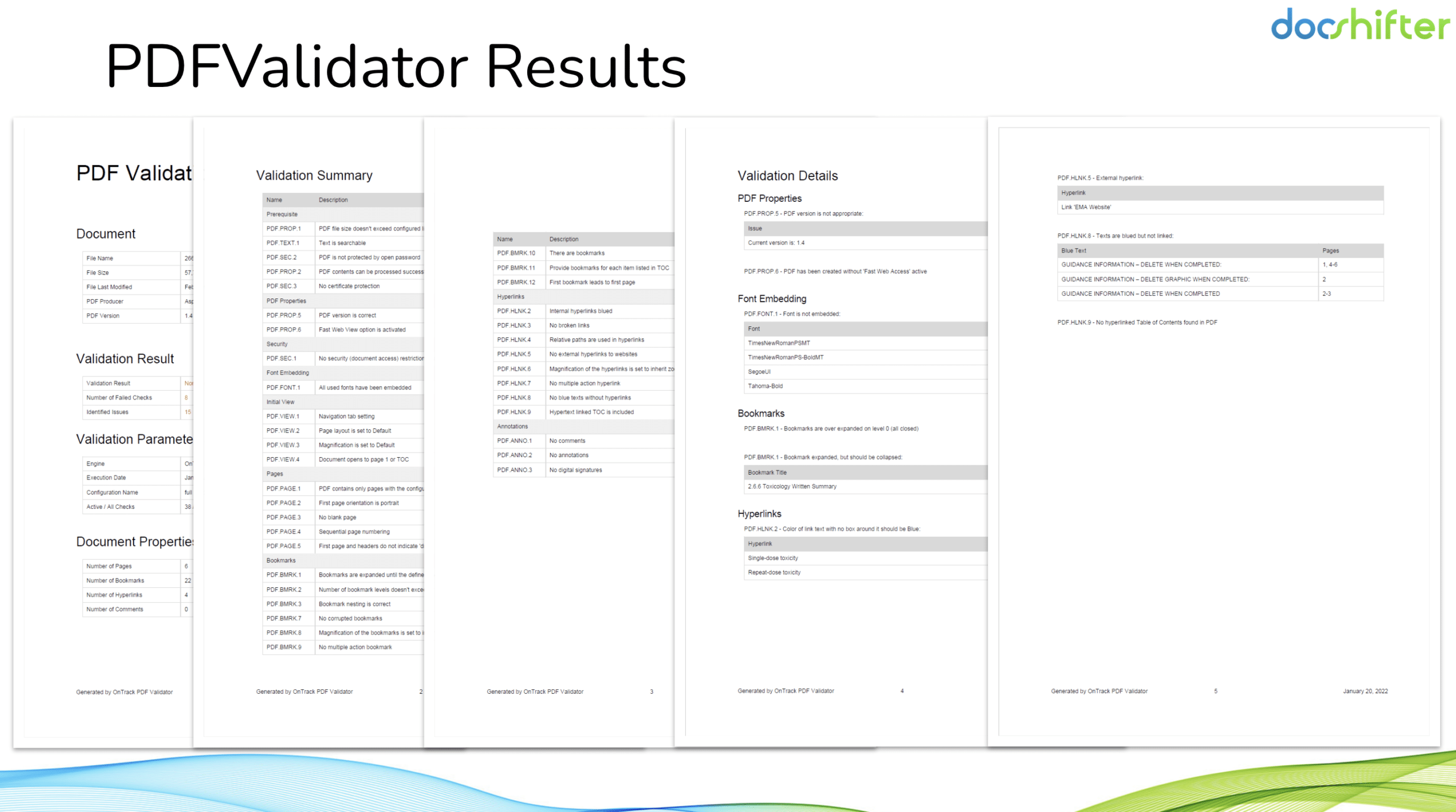
The generated reports provide detailed information on the checks performed, compliance status, resolved and unresolved issues, and the location of each problem: Significantly speeding up your review and correction process, while reducing manual effort and minimising errors.
3. Ribbons, plug-ins, desktop tools are all good, BUT….
Almost everyone in the industry uses ribbons, plug-ins, desktop tools to format and prepare PDFs for submission-readiness. There’s obviously nothing wrong with using these. Everyone in regulatory operations, or anyone responsible for formatting documents, is one way or another familiar with these solutions.
There is however a big limitation to these methods.
They typically work on 1 document at a time. Whether the document contains 10 pages, or 600 pages, makes your job even more difficult. They are very ideal when you only want to work on a couple of pages, but not for a full submission.
Can you imagine creating bookmarks in 600 files in just 2 days like this?
So, what are some ways you can save time and reduce dependency on manual tools?
DocShifter automatically creates fully-formatted, submission-ready PDF documents from your source content: Microsoft Office files, PDFs, images, text files, HTML and XML documents.

What does this mean for you?
It means that any PDF you receive from external sources, or PDFs that are created internally, is fully formatted according to the ICH guidelines. With the correct bookmark structure, hyperlinks, zoom levels, PDF version, with a table of contents (if the document contains more than 5 pages).
The only thing you’d have to do is to run a report, and make sure that the document is formatted accordingly. No need to check document per document, or license or use desktop tools.
Saving you hundreds and thousands of valuable hours.
4. Regulatory operations can not afford to make mistakes. Let DocShifter’s automation take the risk away completely.
Let’s have a look at an example one of our customers experienced in regard to preparing content for regulatory submissions.
This large pharmaceutical company had 2 ways of speeding up the way they prepared documents for submissions. This will probably be recognizable regardless of the company size you are in:
-
Throw more resources at it: Increase the number of people responsible for formatting documents
-
Bring in the right solutions: Bring in automation for document formatting, and let people focus on what matters most: content.
DocShifter’s automation allowed this large pharmaceutical company to significantly process and format more documents in a shorter amount of time.
We are talking about thousands of documents every week, compared to 150 every week.
The result? The regulatory operations team has more time to respond to information requests, seek cross-functional feedback, and spend more time on value-added tasks.