‘Achieve Document Compliance Earlier in Submissions’ — What does this even mean?
-
By DocShifter
- 5 minutes read
But, what do we actually mean by ‘achieve document compliance earlier’?
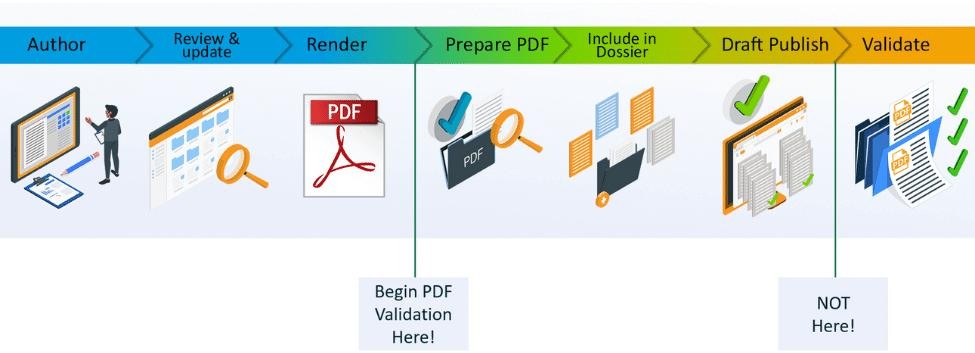
It is actually a pretty simple concept. Instead of validating the PDF files for compliance with the submission itself (after the draft publishing stage), why not create compliant PDFs from the very first moment you create them?
Instead of waiting until the last minute to check if your PDF files are correct, you do it much earlier in the process.
In other words, it’s like making sure your homework is done perfectly as soon as you start it, rather than waiting until the deadline. This way, you ensure that your documents are ready and meet all the necessary rules and regulations, which helps to avoid problems later on.
Now, let’s take a look at the drug development process.
A typical drug development process takes from 5 and 15 years, and documentation is created every step of the way. Submissions are compiled at the end of this timeframe.
Documents are typically only checked for compliance either just prior to or after a draft submission is published.
What if your original source document needs to be updated? Do you really want to be updating source documents, or editing your individual PDFs to ensure each and every document complies with the technical specifications laid out by the Health Authorities? When you are trying to focus on getting the submission out the door! Many of the source documents can be hard to even find.
So, where does the ‘earlier PDF compliance’ come into play?
What we mean by achieving compliance earlier, is to ensure that any issues are identified much earlier in each document’s document’s lifecycle: at the time the source file is saved; or at the time the PDF version is created. It means using a solution to generates fully technically compliant PDFs as soon as the source document is available, and not relying on other tools and plugins to achieve that level of compliance.
Any remaining issues that require an update on the original source document can be identified automatically at source or as the PDF is generated. Rather than manually checking each PDF is submission ready, that check-list can be automated created during the process.
The message we try to get across (and where our customers see the biggest difference) is that by achieving document compliance earlier in this way, you can reduce the risk of missing submission deadlines. Problems can be addressed weeks, months, or even years earlier in the development process and the entire submission creation process simplified.
(Oh by the way, if you are submitting to US FDA – check out our FDA DPF technical specifications checklist)
How does this impact your operations?
When looked at holistically, bringing PDF compliance earlier in regulatory submissions brings huge benefits overall. Not only for time and resource savings, but also in mitigating a lot of (unnecessary) risks when preparing submission content.
1. No last-minute rushing to ensure PDF compliance. The PDFs you create will already be compliant.
2. Accelerate preparing your regulatory submissions by removing manual steps.
3. Identify any remaining issues earlier.
4. Focus on the content, not ensuring technical compliance.
5. Reduce risk of non-compliance and missed deadlines.
A Practical Example: As-Is vs. As-Was
Scenario: Company X is in the final stages of preparing a regulatory submission for a new drug. They have a team of regulatory professionals responsible for compiling all the necessary documents, including clinical trial reports, safety data, and manufacturing information, into a submission package.
Without achieving PDF compliance earlier:
- The team waits until the last minute to check the compliance of their PDF documents, just before the submission deadline.
- They discover several compliance issues, such as incorrect font sizes, missing metadata, and improper formatting, in their PDF files.
- The team scrambles to correct these issues, requiring extensive manual checks and revisions.
- As a result, they miss the submission deadline, delaying the approval process for the new drug and potentially costing the company millions of dollars in lost revenue.
With achieving PDF compliance earlier:
- Company X implements software that automatically generates fully compliant PDFs as soon as the source documents are available.
- Compliance checks are performed throughout the document creation process, flagging any issues for correction in real-time.
- Any remaining compliance issues are addressed immediately, ensuring that all PDF documents are submission-ready.
- The submission package is completed well ahead of the deadline, allowing ample time for review and finalization.
- The regulatory submission is successful, and the new drug receives approval on schedule, enabling Company X to bring it to market sooner and realize revenue earlier.
In this scenario, achieving PDF compliance earlier significantly impacts Company X’s operations by streamlining the submission process, reducing the risk of non-compliance and missed deadlines, and ultimately accelerating the approval of their new drug.
For more information or to have a conversation on how Earlier Document Compliance can save time in your operations, please visit: https://www.docshifter.com/