Everything About Marketing Authorisation Application (MAA) Submissions
-
By DocShifter
- 4 minutes read
In this guide, we will explore everything you need to know about MAA submissions. Stick until the very end, and you will find a link to our Regulatory Professionals Community on LinkedIn where you can find more about everything regulatory in life sciences.
Let’s start: What are MAA Submissions?
An MAA submission is a comprehensive dossier submitted by a pharmaceutical company to regulatory authorities seeking permission to market a new medicinal product. This critical document provides a detailed overview of the drug’s development, including preclinical and clinical data, manufacturing processes, quality control measures, and proposed labeling. MAA submissions are essential for ensuring patient safety and efficacy while facilitating access to innovative treatments.
The process of preparing and submitting an MAA is complex and time-consuming, requiring collaboration between various departments within a pharmaceutical company, as well as external experts. Successful MAA submissions are crucial for bringing new medicines to market and generating revenue for the company.
MAA submissions are relevant to a wide range of stakeholders, including:
- Pharmaceutical companies: Responsible for preparing and submitting MAAs, managing the regulatory process, and ensuring compliance with regulatory requirements.
- Regulatory authorities: Evaluate MAA submissions to assess the safety, efficacy, and quality of the medicinal product before granting marketing authorisation.
- Healthcare professionals: Rely on information provided in MAA submissions to understand the benefits and risks of new treatments.
- Patients: Ultimately benefit from the availability of new and effective medicines.
Key Modules of an MAA Submission
An MAA is typically composed of several key modules. While specific requirements may vary between regulatory authorities, the following modules are commonly included:
Module 1: Administrative Information
This module serves as the foundational element of the MAA, providing essential administrative details about the applicant, the product itself, and the submission process. Key components encompass applicant identification, product description, intended use and target population, and a comprehensive summary of the product’s characteristics.
Module 2: Quality
The quality module is pivotal in demonstrating the product’s consistent production and control. It encompasses a detailed product description, a meticulous outline of the manufacturing process, robust quality control procedures, comprehensive stability data, and precise specifications for packaging and labeling. This module is essential in assuring regulatory authorities of the product’s adherence to stringent quality standards.
Module 3: Non-Clinical Data
This module presents a comprehensive evaluation of the product’s safety and efficacy prior to human testing. It typically includes in-depth assessments of pharmacology, toxicology, pharmacodynamics, and pharmacokinetics. These studies are crucial in identifying potential risks and informing the design of subsequent clinical trials.
Module 4: Clinical Data
The clinical data module is arguably the most extensive section of the MAA, as it provides a detailed account of the product’s safety and efficacy in humans. Key components include a clear description of study design and methodology, a precise characterization of the patient population, comprehensive efficacy and safety data, rigorous statistical analysis, and a thorough benefit-risk assessment. The strength of this module is instrumental in supporting the product’s approval for marketing.
Module 5: Overall Summary
The final module offers a consolidated overview of the entire MAA, providing a cohesive narrative of the product’s development and evaluation. It encapsulates key findings from the quality, non-clinical, and clinical modules, as well as a robust benefit-risk assessment. The overall summary serves as a valuable reference for regulatory authorities and other stakeholders, facilitating a comprehensive understanding of the product’s profile.
MAA Submission Process
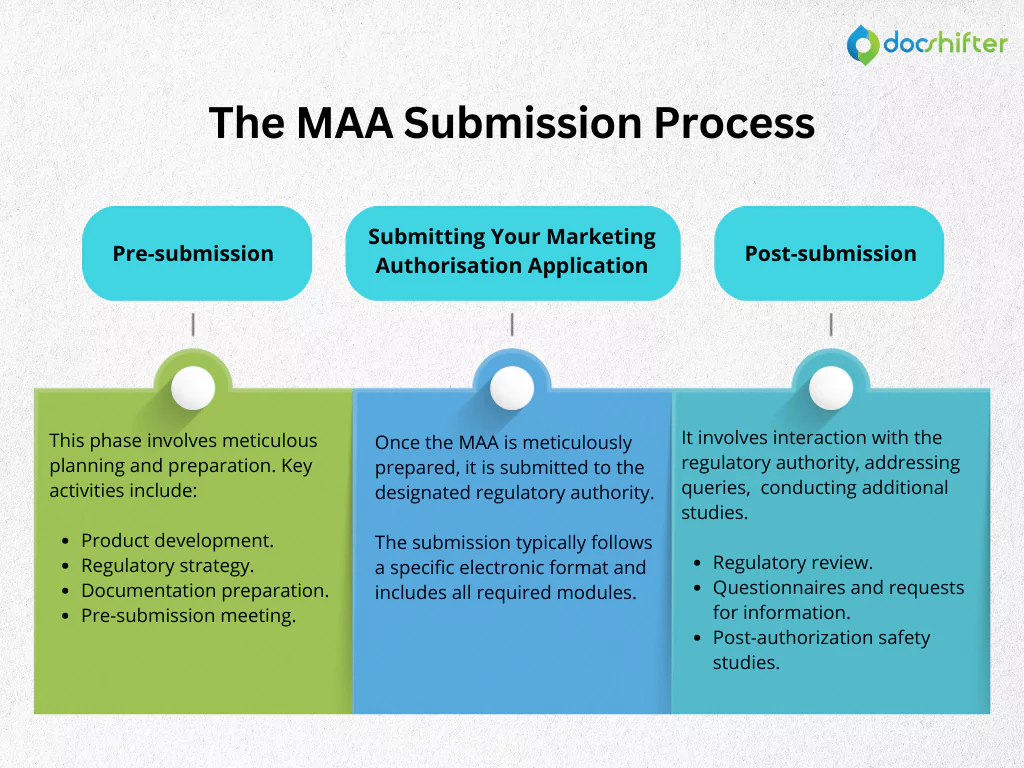
Pre-submission
The pre-submission phase involves meticulous planning and preparation. Key activities include:
- Product development: Research, development, and formulation of the medicinal product.
- Regulatory strategy: Defining the target market, identifying regulatory pathways, and understanding specific requirements.
- Documentation preparation: Assembling comprehensive data and documentation to support the MAA.
- Pre-submission meeting: Optional meeting with regulatory authorities to discuss the development plan and address potential concerns.
Submitting Your Marketing Authorisation Application
Once the MAA is meticulously prepared, it is submitted to the designated regulatory authority. The submission typically follows a specific electronic format and includes all required modules.
Post-submission
The post-submission phase involves interaction with the regulatory authority, addressing queries, and potentially conducting additional studies. Key activities include:
- Regulatory review: Assessment of the MAA by regulatory experts.
- Questionnaires and requests for information: Interaction with the regulatory authority to provide additional data or clarifications.
- Post-authorisation safety studies: Ongoing monitoring of the product’s safety after market authorisation.
Note: The duration of the MAA process can vary significantly depending on the product type, complexity, and the efficiency of the regulatory review.