Practical PDF preparation automation examples and their benefits for you
-
By DocShifter
- 5 minutes read
“ Where do you spend the most time preparing PDFs for use in submissions? ”
This was the starting point for this webinar. We asked this question to more than 1000 people that are involved in PDF preparation for regulatory submissions; of organizations of all sizes.
We wanted to share the results of this survey; as well the learnings from various projects we have been involved in; specifically for the compliant PDF formatting and preparation.
So, what are the challenges? Where do people spend most of their time on?
We’ve gotten interesting answers. And needless to say, the list is pretty expensive. However, here are the highlights:
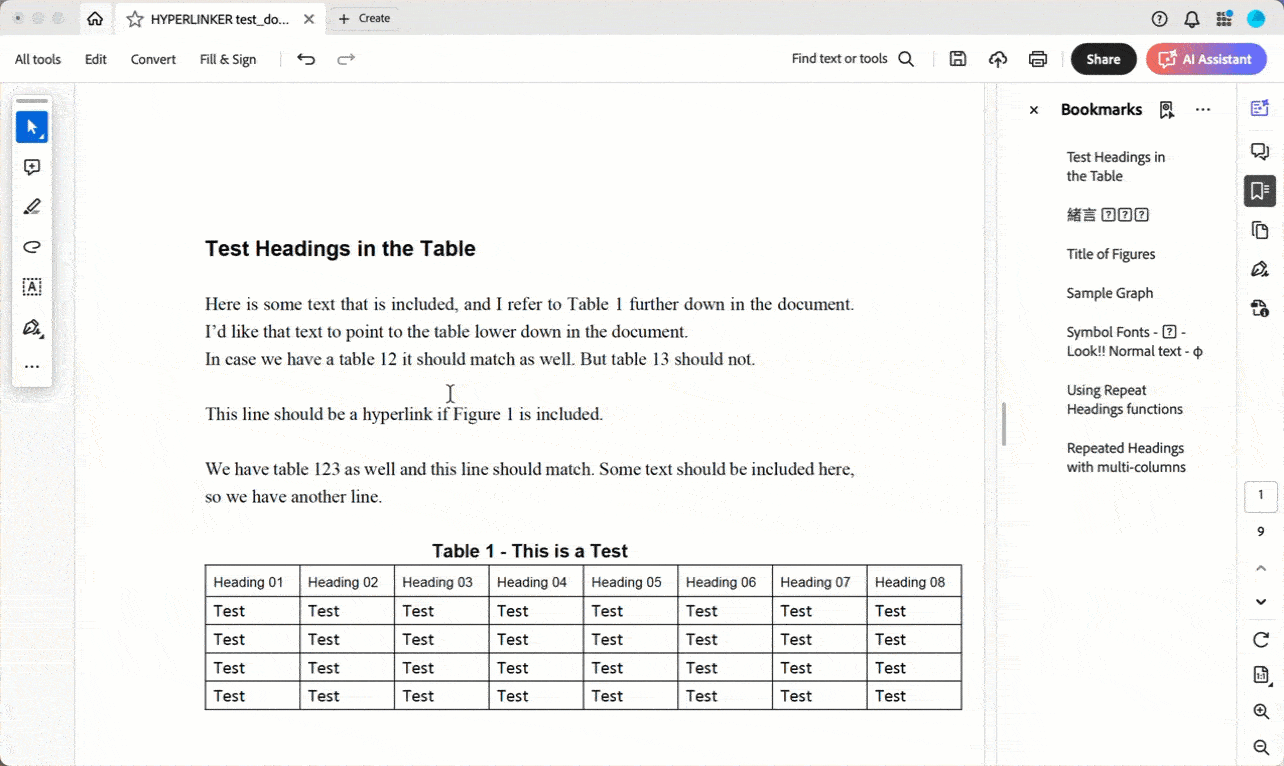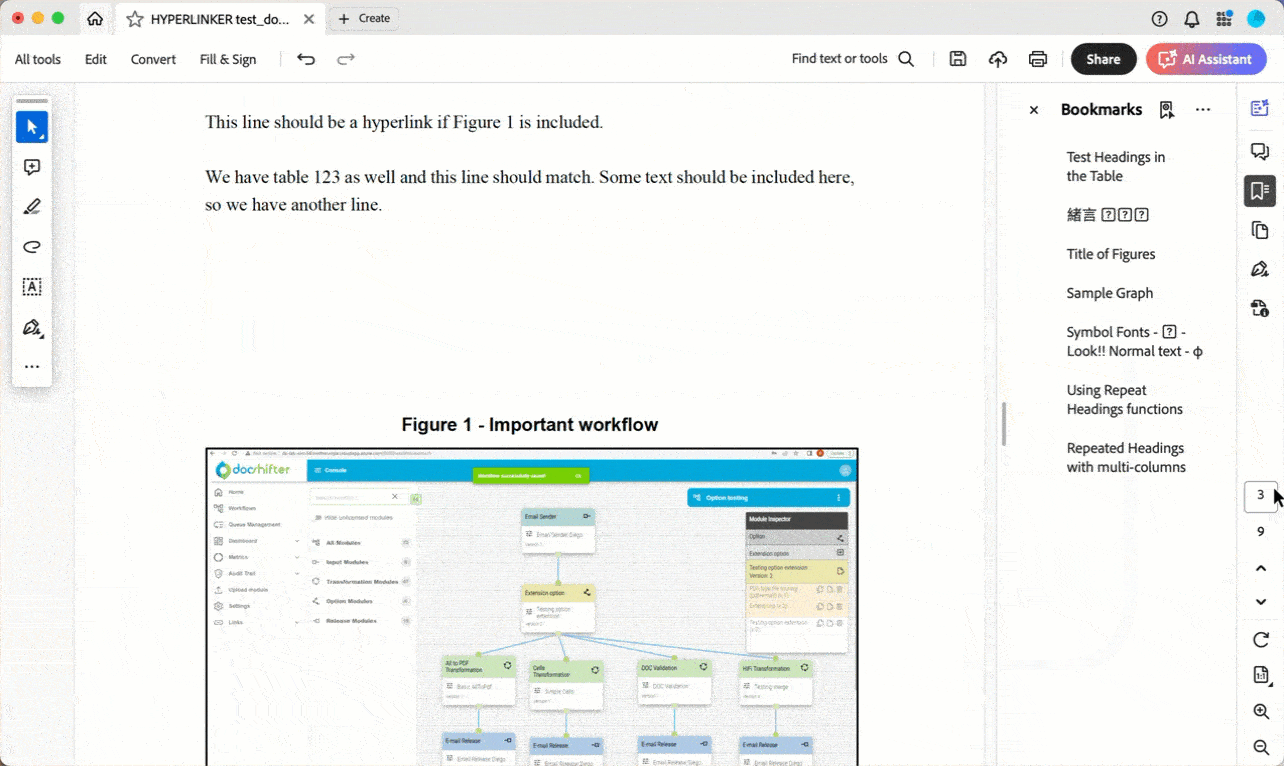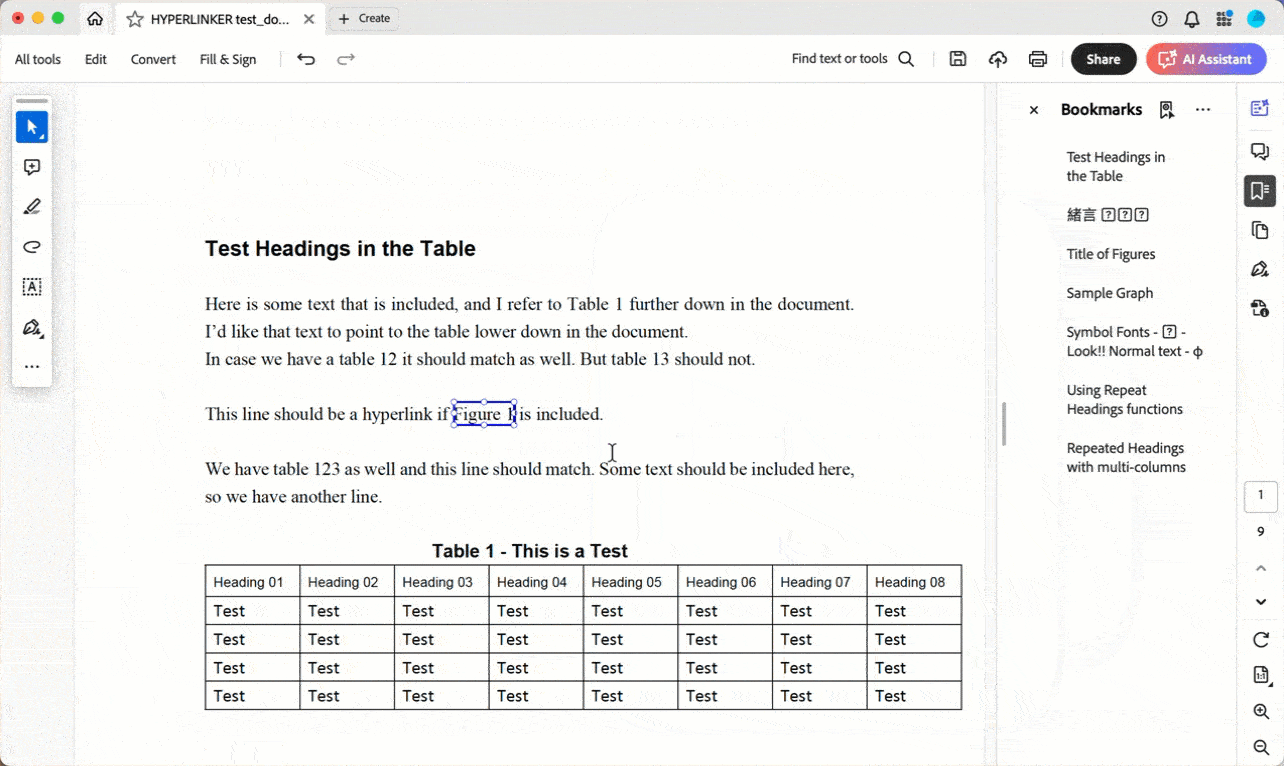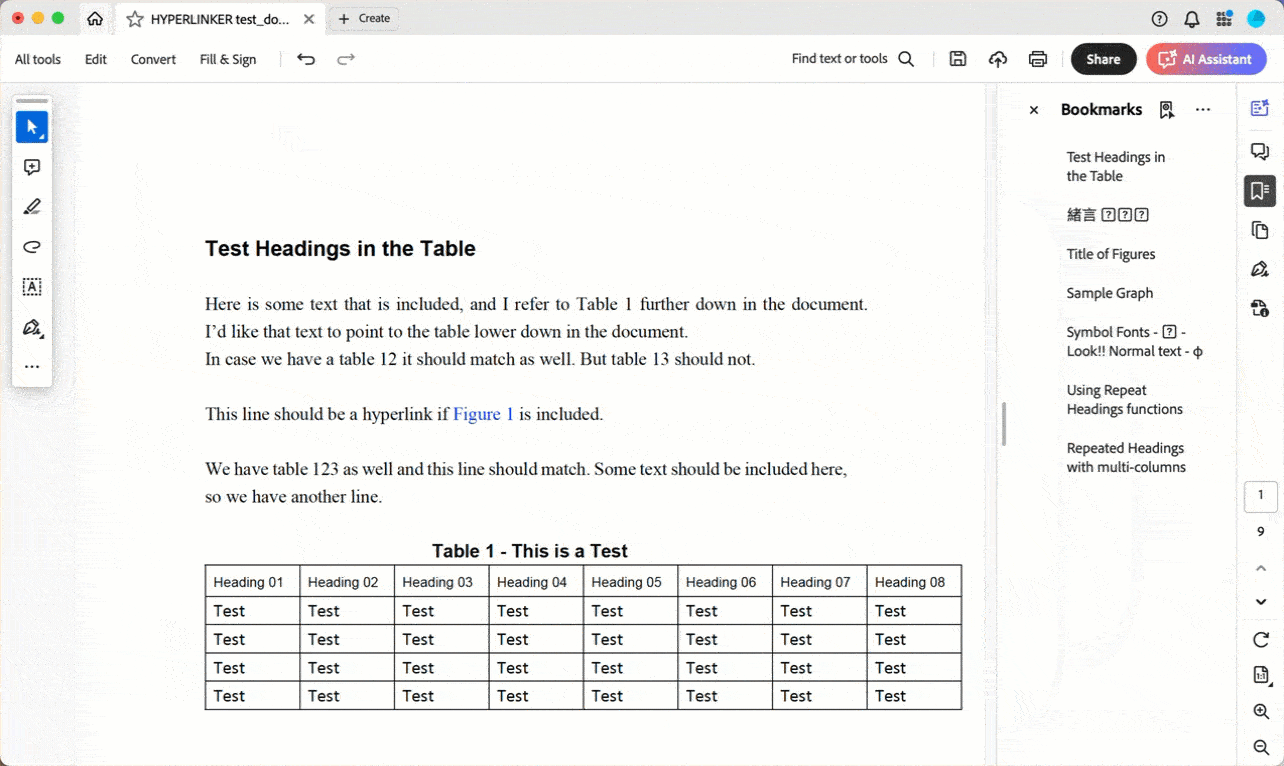
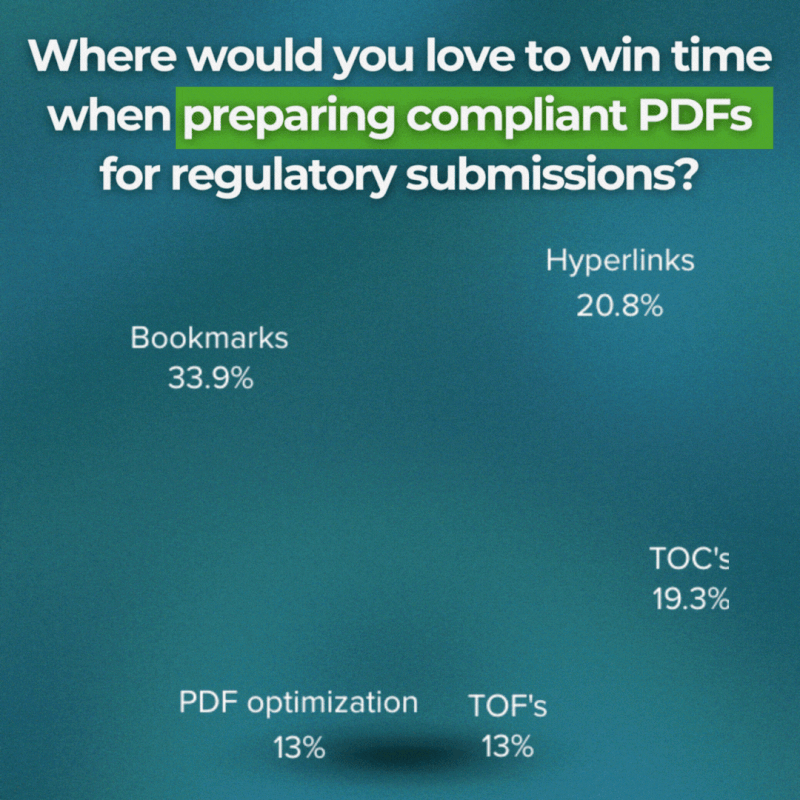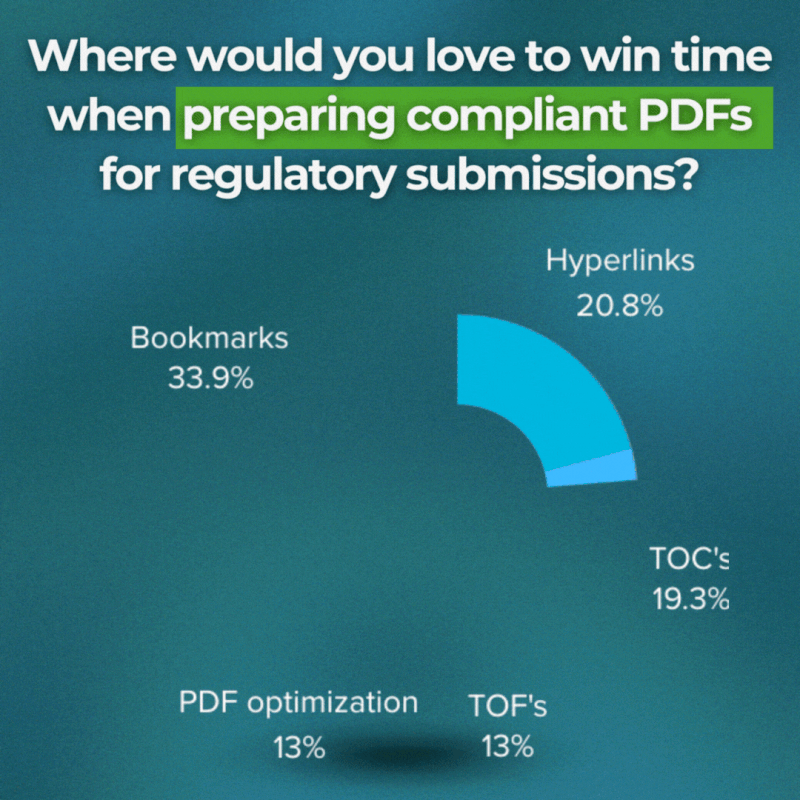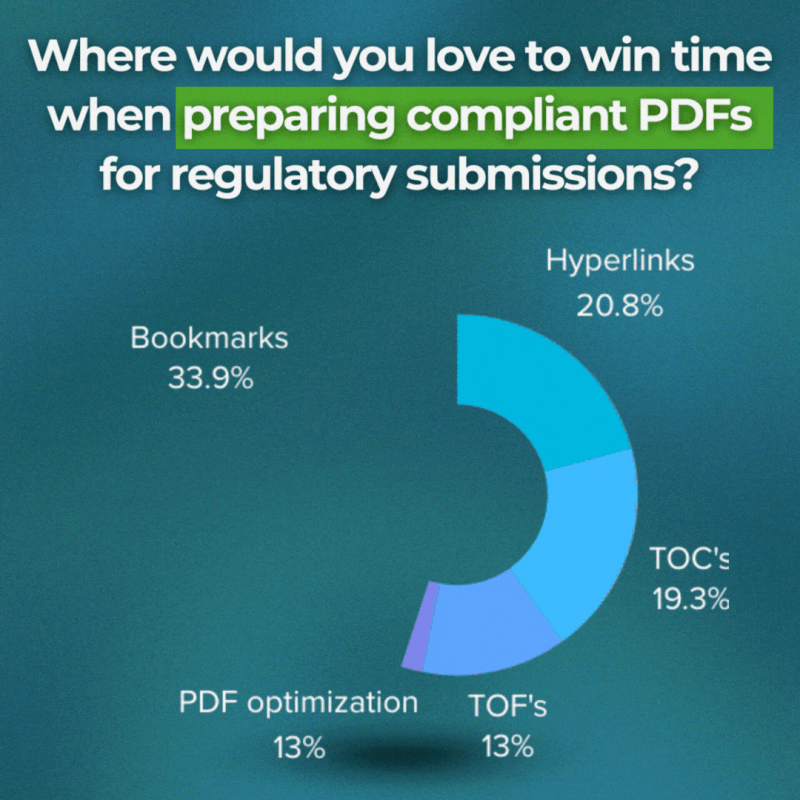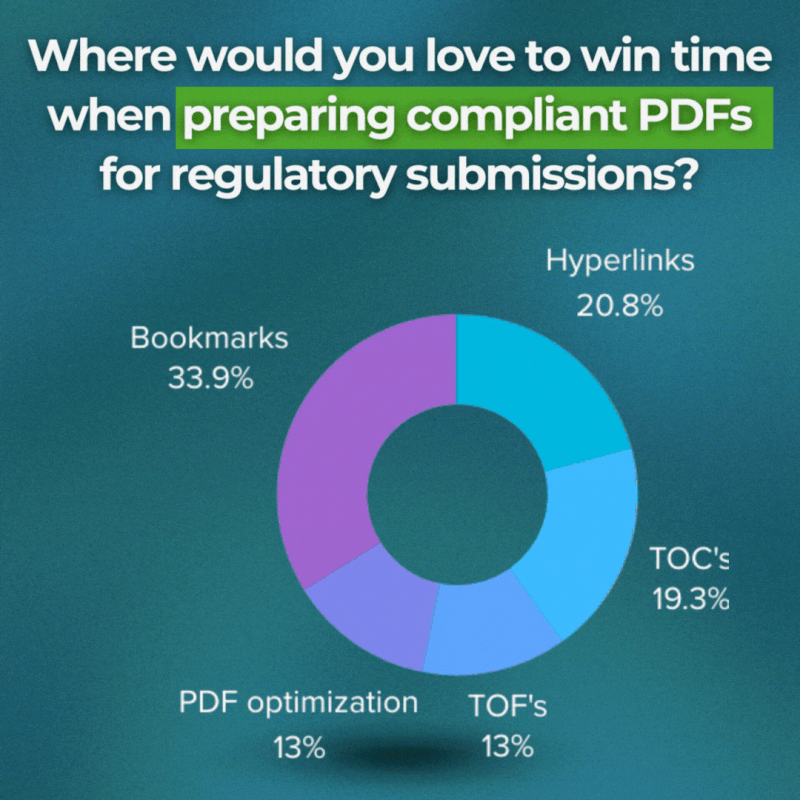
Bookmarks → 33.9%
Hyperlinks → 20.8%
Fonts → 20%
Table of contents → 19.3%
PDF viewing preferences → 17.6%
Table of figures → 13%
PDF optimization → 13%
Why does it take so long to create bookmarks?
We posted about this a couple of weeks ago, and have gotten a lot of engagement. This is very recognizable to so many people that deal with PDFs.
The regulators require navigation-rich PDF documents. How is it that creating hyperlinks is such a time-consuming process?
Dealing with hyperlinks is in the second place according to the poll. In this GIF, we are really displaying the extreme manual hyperlinking scenario.
Hyperlinks are tricky because they need to:
- Point to the right place within the document
- Inherit the correct zoom level
- Have the right color
If you have to ensure hyperlink compliance in your PDFs, you need to do a lot of right clicks in your PDF files.
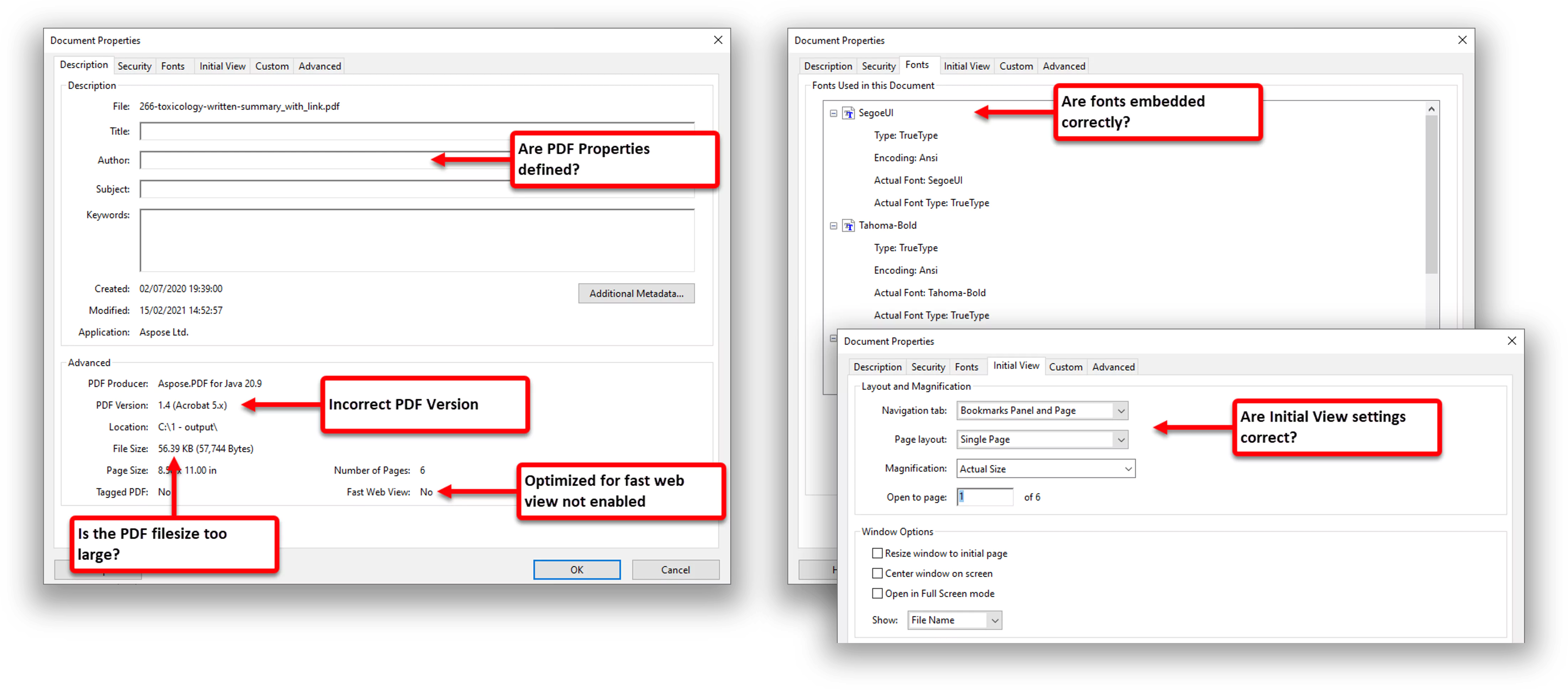
Identifying other issues in PDF files before the publishing stage
Is my PDF version correct?
Is the PDF file size too large?
Are my PDF properties defined?
Is my PDF optimized for fast web view?
Are my initial view settings correct?
Are my fonts embedded correctly?
Is there a table of contents, if my PDF contains more than 5 pages?
Does my PDF contain annotations and comments?
Are the photographs at least 600 dpi?
There are a lot of elements you still need to look in your PDF files to achieve technical PDF compliance.
Even though some of these things can be handled at the publishing stage, you would ideally want to tackle everything way earlier to win valuable time and resources during time-critical submissions.
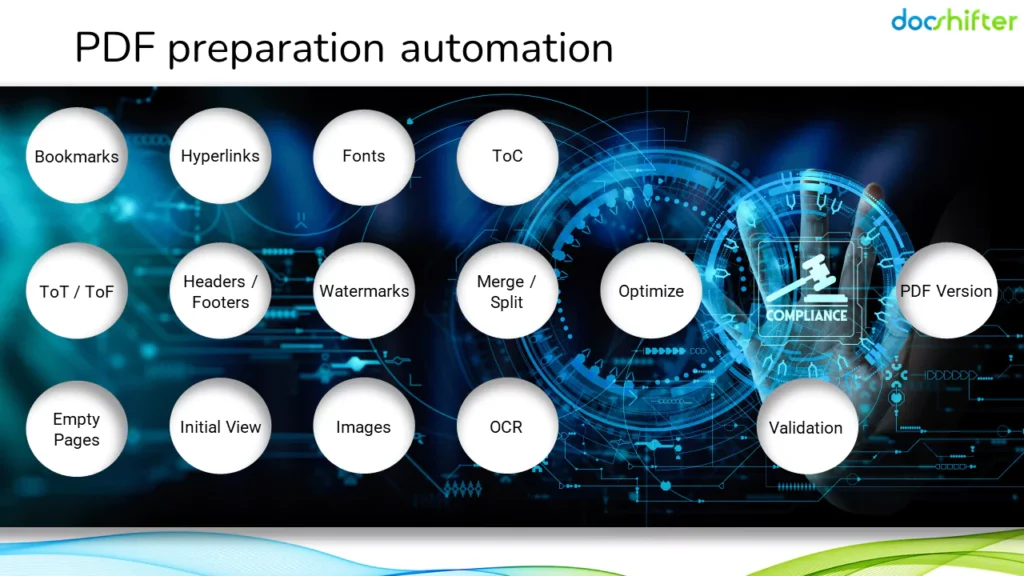
So, let’s talk about automating PDF preparation for regulatory submissions. And some real-life automation examples.
Every single element that helps you achieve technical PDF compliance can be automated. From bookmarks, hyperlinks, fonts, PDF optimization, initial view settings, removing empty pages, to PDF version; watermarks and so on.
Even if you save 5 minutes on bookmarks, hyperlinks, table of contents, watermarks, images and other elements, 5 minutes per 100 documents is a tremendous time saving.
Here are some PDF preparation automation scenarios.
- You want to validate a PDF file, and check whether it complies with the HA requirements: https://youtu.be/lFN0pFKD2ZM?si=4T5G7bL4kjFBKIRz&t=475
- You want to fix PDF files, in case your PDFs do not comply with the HA specifications: https://youtu.be/lFN0pFKD2ZM?si=-8HliO7Ke7hvjnyM&t=740
- You want to automatically create internal and external links using automation: https://youtu.be/lFN0pFKD2ZM?si=TG3cUVKzWr10Muau&t=882
- You want to generate FDA, PMDA and EMA compliant PDFs from a single source document: https://youtu.be/lFN0pFKD2ZM?si=bzIRW5Nu92EG3Wa0&t=1057
- You want to bookmark a PDF where the source content does not contain any bookmarks: https://youtu.be/lFN0pFKD2ZM?si=VfYpKL1v6KaB2t0_&t=1338
How about the PDFs we receive from external stakeholders? How can we ensure their compliance, automatically?
“Even though we have clear templates in place, not everyone uses those templates. Even if they do, documents are created on various platforms and devices; so differences between documents are inevitable. These differences need to be checked and corrected before they are included in the dossier.”
What if there was a way for you to see what is wrong with your PDF files with the click of a button? To automatically check PDFs regardless of how they were created, and tell you where to look at for issues?
All these questions we tackled above; whether your PDFs meet the technical requirements of health authorities for inclusion in dossiers, can be shown to you in a clear report.
An automated compliance checker for PDF files. This is exactly what PDFValidator from DocShifter does.
Configurable for internal and external guidelines, PDFValidator checks and fixes formatting and styling issues in your PDF files.
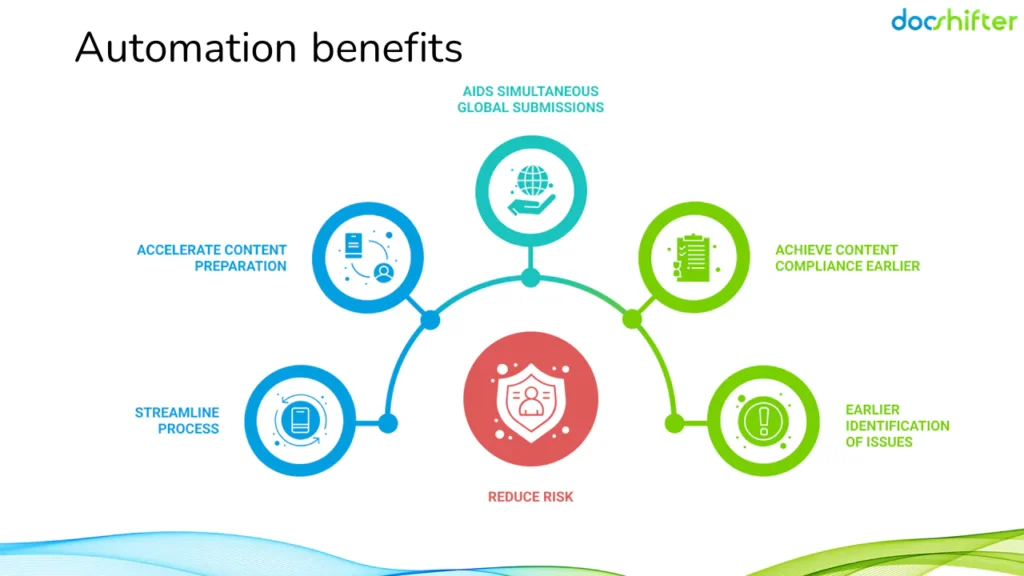
Automating our PDF preparation process. What is in it for us?
Ultimately, you want to increase the overall regulatory compliance and efficiency. Accelerate time to market. Automating PDF preparation can help you achieve these; and more.
Increased Efficiency: Automating repetitive tasks like adding bookmarks, hyperlinks, embedding fonts, and generating tables of content will free up your time and that of your team. This allows you to focus on more critical aspects of the regulatory submission process.
Reduced Errors: Manual data entry is a leading cause of errors in PDFs. Automation minimizes these errors by ensuring consistency and accuracy in the preparation process.
Faster Submission Times: With automation speeding up the PDF prep stage, you can potentially submit your regulatory documents faster, potentially meeting deadlines quicker.
Improved Compliance: Consistent and accurate PDFs prepared through automation can help ensure your submissions meet the formatting and content requirements of regulatory bodies.
For more information and for all your questions, please visit https://www.docshifter.com/