Practical PDF preparation examples to make your life easier
-
By DocShifter
- 4 minutes read
The Role of PDFs in Regulatory Submissions: Navigating Challenges and Ensuring Compliance in the Life Sciences Industry
PDFs play a crucial role in the life sciences industry, especially when it comes to regulatory submissions, documentation, and communication.
Regulatory bodies like the FDA and EMA rely on PDFs as the standard format for submissions due to their reliability, consistency, and versatility in handling various content types, including text, images, and data tables.
According to the FDA’s guidance on electronic submissions, PDFs are required for all document-based submissions to ensure consistency and accessibility across various review platforms. In fact, over 90% of electronic submissions to the FDA are made in PDF format, underscoring the format’s critical role in regulatory communication.
If you are also submitting to the FDA but aren’t sure about the FDA’s PDF specification requirements, please visit this article + download the free FDA PDF checklist:

Common Challenges in Managing PDFs in the Life Sciences Industry
Despite their importance, managing PDFs in the life sciences industry comes with its own set of challenges. These include ensuring consistency across different documents, maintaining the integrity of data when converting files, and navigating the complexities of regulatory requirements. A report by Deloitte highlights that nearly 70% of life sciences companies face challenges related to document management and regulatory compliance, particularly in the context of PDF submissions. These challenges can lead to inefficiencies and potential compliance risks if not properly managed.
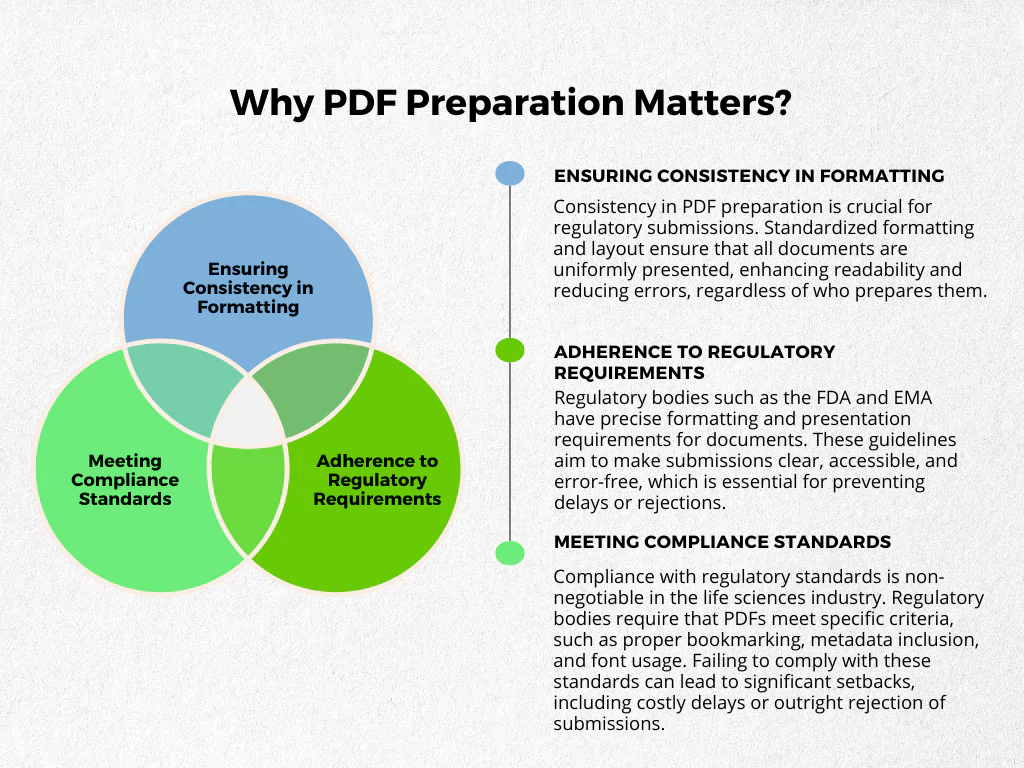
Understanding the Basics: Why PDF Preparation Matters?
How can you make your life easier when dealing with PDFs? Here are some practical examples.
Example 1: Automated Bookmarking
Automated bookmarking allows to automatically create bookmarks within PDF documents, which is crucial for efficient navigation and document management.
After all, no one wants to create bookmarks manually. [This is how it looks like if you do.]
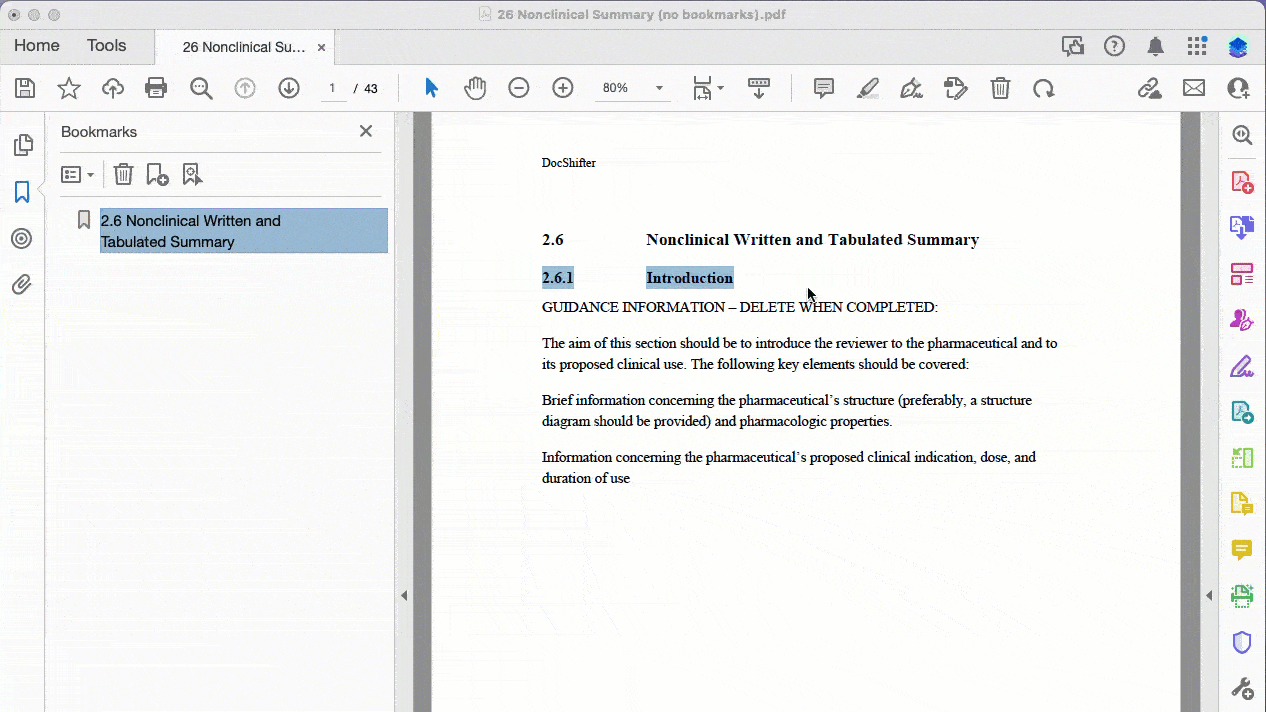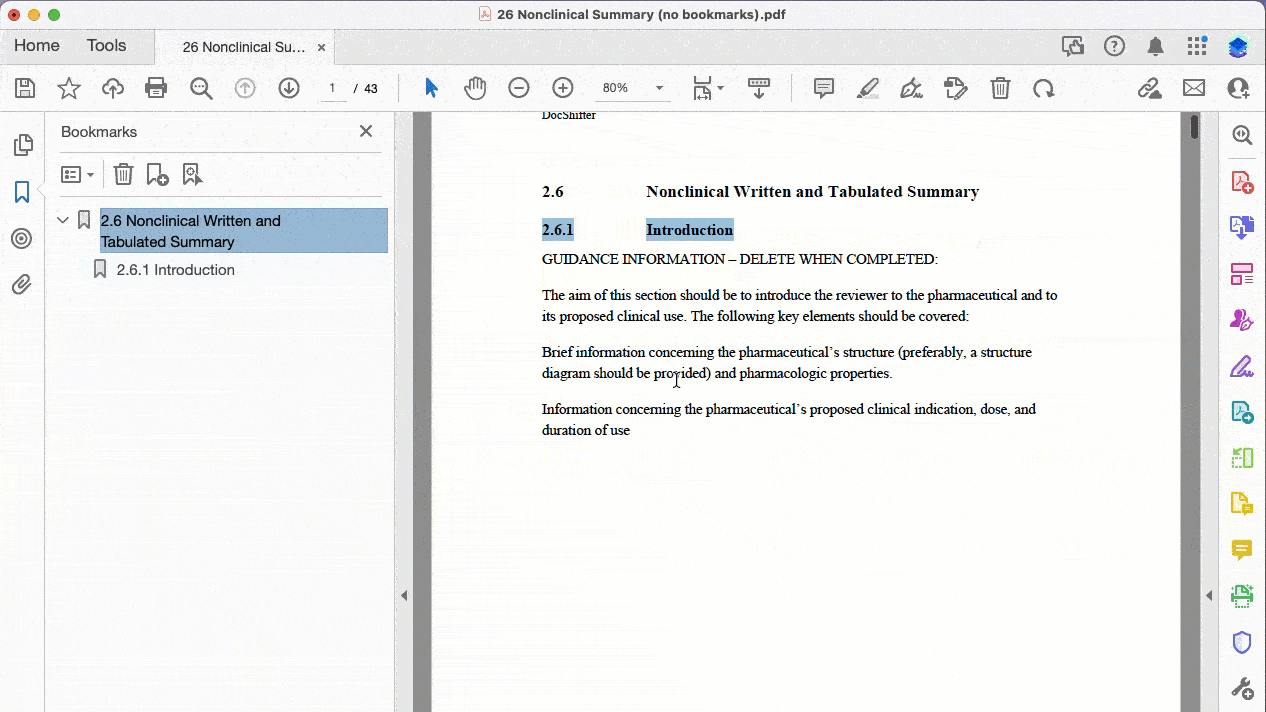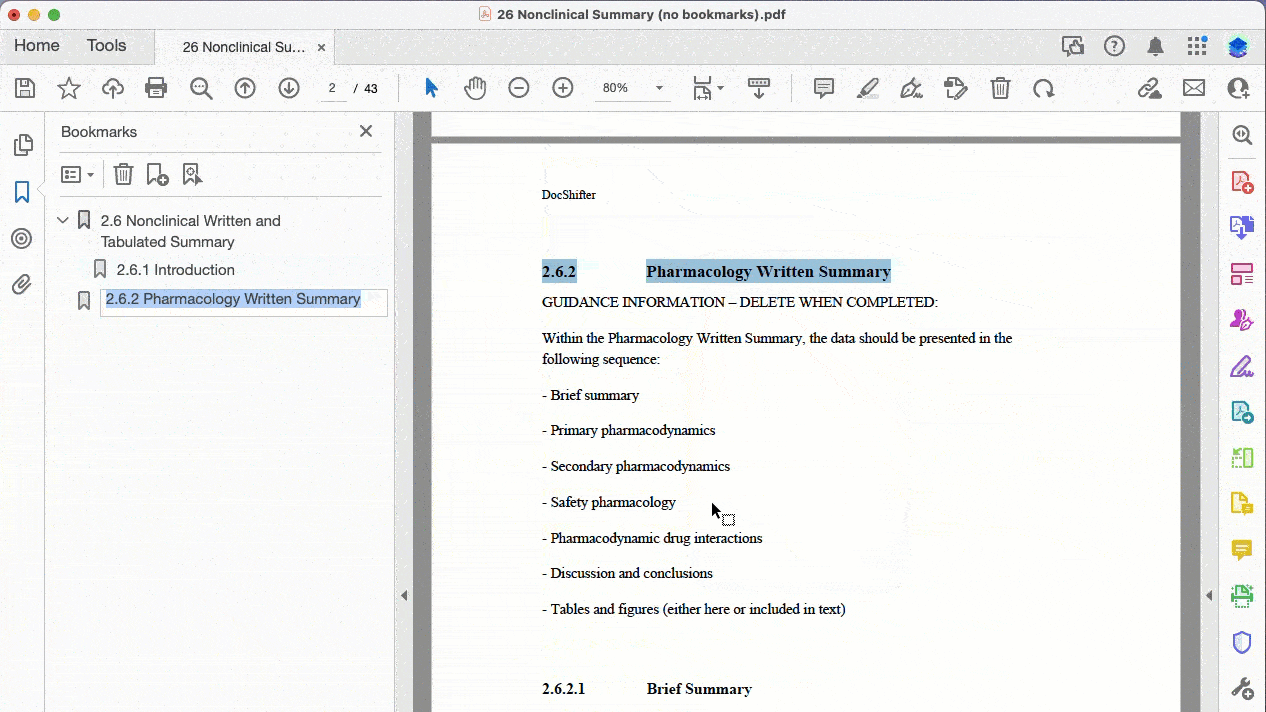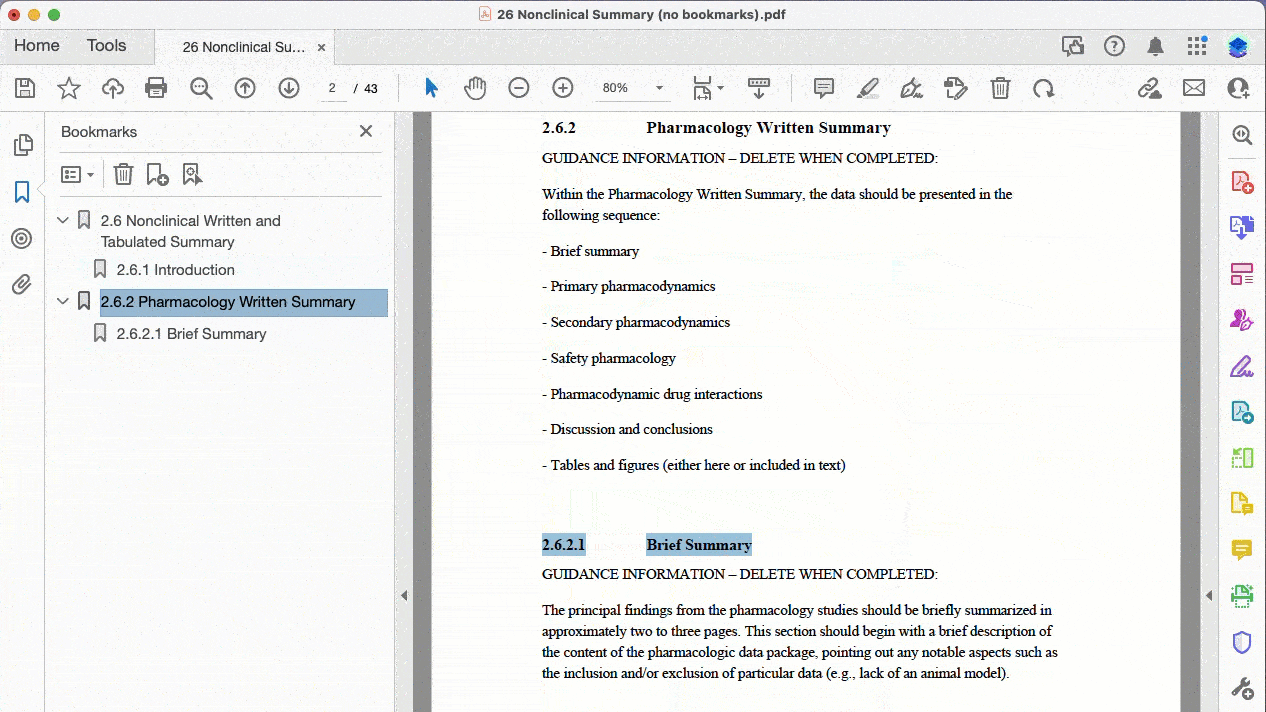
With DocShifter you can create bookmarks in various ways:
-
Generate bookmarks from any styles, including your own custom styles
-
Ensure hyperlinks are consistently formatted and styled
-
Set magnification and zoom level for bookmarks and hyperlinks
-
Automatically create bookmarks for PDFs that have no bookmarks (based on text font, size, and more)
Here’s a practical example of bookmarking a PDF where the source does not contain any bookmarks:
Example 2: Document Merging & Report Generation
Document merging and report generation with DocShifter simplifies the process of combining multiple documents into a single, cohesive PDF and generating reports that are compliant with industry standards. Here’s how it works:
1. Document Merging:
Automatically merge documents of various formats (e.g., Word, Excel, PDFs, image files, etc.) into a single PDF. This is useful when you need to bring together different pieces of content into one document. During the merging process, DocShifter ensures that formatting is always consistent across all documents, preserving headers, footers, and other important PDF elements.
You can create one or multi-volume reports; split based on page numbers or file size; and the output can be fully submission-ready if desired.
See automated report generation with real examples here in this short video:
Example 3: PDF Validation & Quality Checks
Is my PDF document ready for publishing?
Are the PDFs we receive meet our internal guidelines? Are the PDFs we receive meet our internal guidelines?
What are the missing elements from my PDF files?
PDF validation is here to answer these questions.
PDF validation checks ensures that your PDF documents meet the required standards for your regulatory submissions or internal use. These checks help maintain compliance, document quality, and accessibility.
DocShifter checks & validates PDFs against specific regulatory standards such as FDA, EMA, and PMDA requirements. It ensures that the document meets all necessary criteria, including formatting, bookmarking, and metadata requirements.
Here’s how it looks:
Conclusion
In the life sciences industry, the role of PDFs in regulatory submissions is both indispensable and complex. The format’s reliability and versatility make it the standard for submissions to regulatory bodies such as the FDA and EMA. However, managing PDFs effectively presents significant challenges, from maintaining consistency to ensuring compliance with detailed regulatory requirements.
Addressing these challenges through strategic PDF preparation is crucial. Implementing standardized formatting protocols, adhering to regulatory guidelines, and leveraging advanced tools like DocShifter can streamline the submission process and mitigate compliance risks. Practical examples, such as automated bookmarking, document merging, and PDF validation, illustrate how modern tools can simplify these tasks and enhance document quality.
By prioritizing effective PDF preparation practices and utilizing advanced tools, organizations can not only meet but exceed regulatory expectations, ultimately facilitating smoother submissions and more efficient operations.