Navigating the Regulatory Submission Process
-
By DocShifter
- 8 minutes read
Regulatory submissions are a very critical milestone in the life sciences industry. They are literally the translation of (mostly) 10 years’ worth of R&D work into success.
Therefore, navigating regulatory submissions is a critical and often complex hurdle.
The regulatory submissions process involves 5 main stages, being:
- Pre-submission
- Application preparation
- Submission & review
- Addressing deficiencies
- Approval
We aim to equip you with a clear understanding of what regulatory submissions are, why they matter, and the key steps involved in securing approval for your innovative product.
What are Regulatory Submissions?
Think of regulatory submissions as the gateway for your groundbreaking drug, medical device, or biotherapeutic to reach patients.
These comprehensive packages – a.k.a. regulatory submission or a regulatory dossier – are submitted to regulatory bodies like the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
They showcase your product’s safety, efficacy, and quality, demonstrating compliance with stringent regulations. Overall, there are nine main submissions that can be made to regulatory bodies, falling into three categories. They are:
Drug applications:
- New Drug Applications (NDA)
- Investigational New Drug Applications (IND)
- Abbreviated New Drug Applications (ANDA)
Medical Device Submissions:
- 510(k) Premarket Notification (510k)
- Pre-market Approval (PMA)
Other submissions:
- Bio Licenses Application (BLA)
- Marketing Authorization Application (MAA)
- Clinical Trial Application (CTA)
Why Do They Matter?
Regulatory submissions are essential for ensuring the safety and effectiveness of products impacting human health. They contain all the necessary information for a regulator to judge the efficacy, safety. Submissions protect patients as well, building trust in your innovation, and paving the way for market access. Submissions ultimately bring your life-changing solutions to those who need them most.
The Regulatory Submission Process
Now that you have a bit of background knowledge, it’s time to get into the regulatory submission process. Below is a high-level overview of the five-step journey.
- Pre-submission: Planning and gathering data, understanding requirements, and engaging with regulators.
- Application Preparation: Compiling a detailed dossier with scientific data, manufacturing information, and labeling.
- Submission & Review: Submitting the dossier electronically and facing regulatory scrutiny.
- Addressing Deficiencies: Responding to agency questions and addressing any identified issues.
- Approval or Not? Receiving a final decision, with potential post-approval monitoring and reporting.
Getting Prepared
In this section, we are going to talk about the steps you need to take to launch your new product. And for this, a smooth regulatory approval process is critical. Here’s how to get your organization prepared for regulatory success.
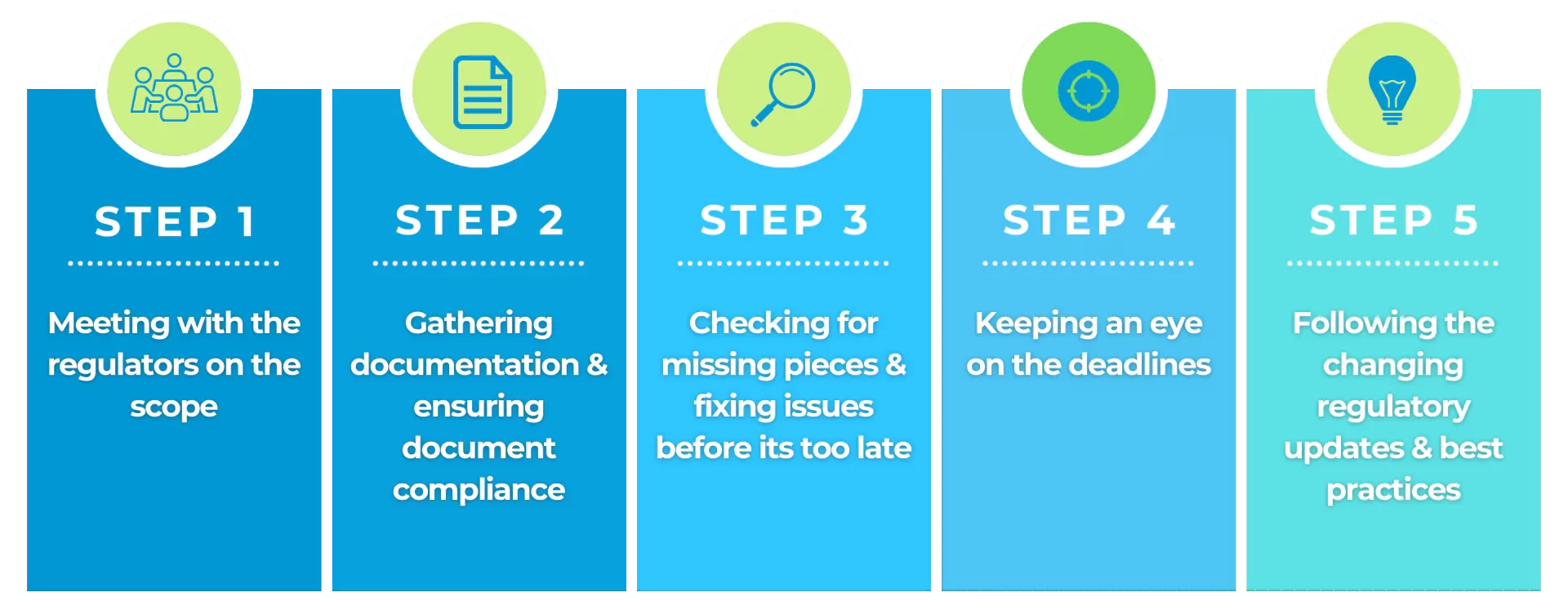
Step 1: Talk it out with the regulators
Before submitting your documentation, chat with the FDA or EMA (think of it like getting insider tips). Discuss your product, ask questions, and get clear on their expectations. This early chat can save you time and headaches later.
Step 2: Gather Your Documentation
Collect all the required documents, like clinical trial data, manufacturing details, and clear labeling. Think of it like packing your suitcase for a big trip – you don’t want to forget anything important. Especially when every day is costing you lots and lots of time and money.
Please remember that you can not just send the documentation in any format you want. There are specific guidelines you need to follow on how the documentation needs to look like.
These guidelines are set out by the ICH – International Council of Harmonization. For submissions in the electronic common document format (eCTD), the specifications for submission formats can be found here:
Another key piece is to understand what steps all this documentation typically needs to go through. This video from Paul Ireland explains the step-by-step document lifecycle for your documents for regulatory submissions.
Step 3: Check for Missing Pieces
Do a quick self-assessment before submitting. Are there any gaps in your data, missing info, or things that don’t quite fit the regulations? Fixing these now means less back-and-forth later.
There is submission validation software available to check whether your submission is complete, compliant, and ready to be shared with a health authority. Technology to the rescue again.
Step 4: Deadlines – Not Set in Stone
While there aren’t specific deadlines, knowing typical review times for your submission type helps you plan.
Step 5: Stay in the Know
Regulations change and evolve for many reasons. It is important to keep yourself up to date with the latest updates and best practices. There are many conferences, webinars, seminars or e-books available.
A few sources you might want to follow are the conferences from the DIA (Drug Information Association) and RAPS (Regulatory Affairs Professionals Society).
Submission Challenges
It is always good to lay out the challenges you may face.
Common challenges that organizations face as they move their way through the regulatory submission process include:
Country-Specific Quagmires
- Challenge: Each nation has its own unique set of regulations and submission requirements. Juggling these differences, especially between regions like the US and Europe, can be daunting.
- Solution: Proactive research is key. Partner with experts familiar with your target markets to stay abreast of evolving regulations and navigate specific requirements effectively.
Lack of a Strategic Compass
- Challenge: Diving head first without a clear strategy can lead to delays and inefficiencies. Companies may lack a defined approach, failing to conduct pre-submission meetings with regulatory agencies for valuable guidance.
- Solution: Develop a robust regulatory strategy from the outset. Consider factors like target markets, competitor landscape, unmet medical needs, first-in-market potential, and initial target indications. Pre-submission meetings with agencies can provide invaluable insights and shape your strategy.
Inconsistent Data Storytelling
- Challenge: Inconsistent data and a fragmented narrative weaken your submission. Companies may struggle with inadequate context description or inconsistencies arising from different subject-matter experts authoring different sections.
- Solution: Ensure a unified voice and consistent data throughout your submission. Establish clear guidelines and central coordination to ensure all pieces seamlessly contribute to a compelling narrative.
Submission Expertise Gap
- Challenge: Hiring and retaining regulatory experts can be difficult, especially with changing business strategies and emerging product types. This can lead to skill gaps within submission teams.
- Solution: Invest in training and development for existing team members. Outsourcing specific tasks to experts or partnering with regulatory consultancies to fill temporary gaps, might be a solution for your regulatory submissions.
Regulatory Review
You’ve submitted your application, now comes the wait… the regulatory review process. The regulatory review process, just like a regulatory submission, depends on the region you submit the dossier to.
Every regulatory body has its own review process, with varying timelines and milestones. We can’t cover them all here, but here are some helpful links to get you started:
|
Country/Region |
Regulatory Body |
Official Review Process |
Focus |
|
United States |
Food and Drug Administration (FDA) |
Drugs, medical devices, biologics, food, cosmetics, tobacco |
|
|
European Union |
European Medicines Agency (EMA) |
Medicinal products for human and veterinary use |
|
|
Japan |
Pharmaceuticals and Medical Devices Agency (PMDA) |
Pharmaceuticals, medical devices, regenerative medicine |
|
|
Canada |
Health Canada (HC) |
Drugs, medical devices, food, natural health products, veterinary drugs |
Think of these as road maps, with key milestones marking progress along the way.
Staying in Touch
Communication is key during the review. Engage proactively with the agency, responding promptly to questions and clarifying any concerns. Remember, they’re your partners in ensuring patient safety.
Possible Destinations
There are three main outcomes for your regulatory submission. They are:
- Approval: Your product gets the green light. That is not just fantastic news for your company and your team, but it’s also fantastic news for the patients. Congrats! Celebrate responsibly and prepare for post-approval monitoring.
- Request for Additional Information (RAI): Not a rejection, just a hurdle. Address the RAI thoroughly and promptly to keep moving forward.
- Rejection: While disappointing, it’s not the end. Understand the reasons and consider resubmitting with the necessary improvements.
For more on refusal to file (RTF), check out our guide, which specifically focuses on technical document compliance.
Remember: The review process is crucial for ensuring safety and efficacy. Embrace it as an opportunity to polish your product and build trust with regulatory bodies. By understanding the process, communicating effectively, and staying prepared, you’ll navigate this stage with confidence and pave the way for bringing your innovation to the world.
More Automation Available in Today’s Market to Help with Your Regulatory Submissions
Today, more and more software solutions are available on the market to help with every step of your submission.
Though they might all offer different things, their objective is the same: save you valuable time and resources during the cumbersome regulatory submission process.
Software solutions like DocShifter can ensure that every single document that is added to your regulatory submission, is fully compliant with the requirements of the health authority you are submitting to.
By being open, prepared, and embracing helpful technology, you can breeze through the submission process and get your amazing product out there faster.
Remember, a smooth submission paves the way for a bright future filled with helping people!