eCTD 4.0: Key Changes & Impact on Submission Content Preparation
-
By Othmane Achir
- 8 minutes read
 Who is (unfortunately) old enough to remember this?
Who is (unfortunately) old enough to remember this?
Date: March 2007.
A submission. 500 binders for Module 5, 60 binders for Module 4, 30 binders for Module 3, 10 binders for Module 2, and 2 or 3 binders for Module 1.
In total 600 binders, each 300-350 pages totaling about 200,000 pages. Each box contains 5 binders, a pallet contains 18 boxes, so my guess is you are looking at 3 (perhaps 4) copies.
Thank you electronic submissions for taking this burden away. And thank you eCTD 4.0 for further improving the standards. Now, let’s take a deeper look at eCTD 4.0.
What is eCTD 4.0?
eCTD 4.0 is based on the Health Level Seven (HL7) standard called RPS (Regulated Product Submission). This standard focuses on simplifying the processing and review of regulated product information rather than the actual content itself, which will continue to develop with each iteration of the eCTD. RPS was also designed to be used in other sectors to gain approval for medical devices, animal health and other regulated products in the future.
The primary goals of eCTD 4.0 are to:
- Implement changes that speed up the regulatory submission process.
- Enhance how agencies and sponsors communicate.
- Improve global harmonization of the format.
Current eCTD 4.0 Implementation Status
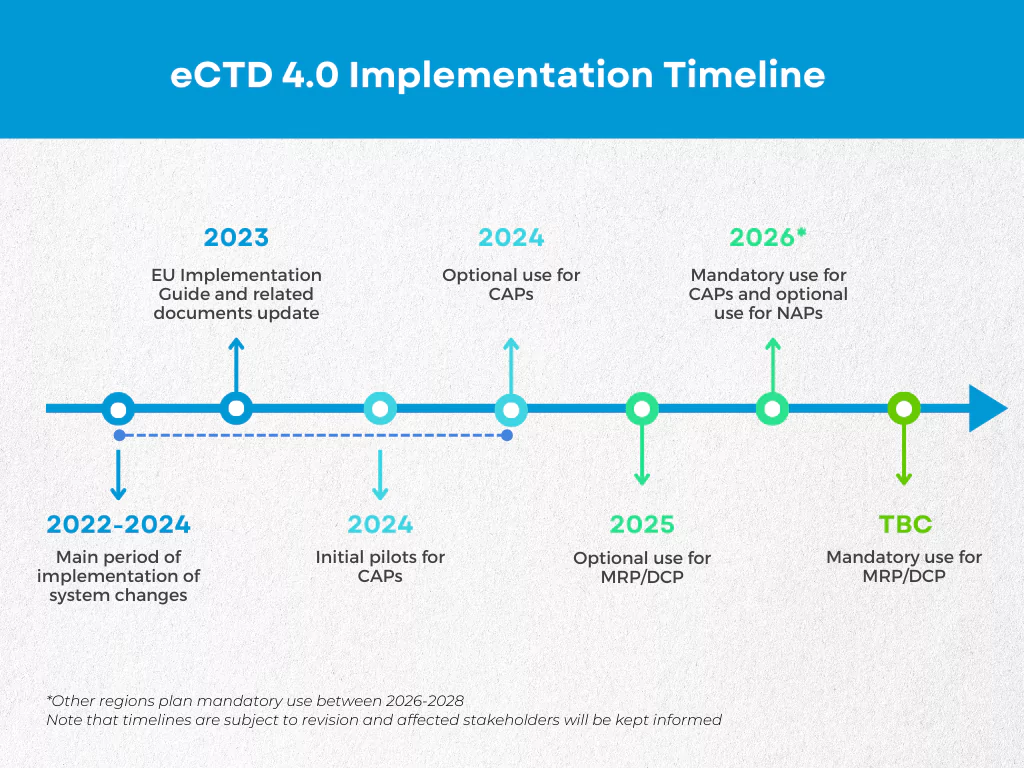
Despite numerous delays (the FDA for example planned to begin their initial pilot in 2015), a number of health authorities have completed, started or planned pilots for their implementation of eCTD 4.0.
In addition to the delays, not all aspects of eCTD 4.0 are expected to be implemented from day one. The two-way communication, which is seen as a key component of the new version, will not be implemented initially in the US, for example. Future phases will incorporate this capability and processes to continue applications initially submitted in the 3.2.2 spec in the 4.0 specification. These will be critical to the long-term success of the format.
eCTD 4.0 Implementation Timeline
Key Changes in eCTD 4.0
While there will be some process changes required by the sponsors, most of the burden will be placed on the publishing and reviewer vendors to adapt their solutions to the new standards. There will be more reliance on technology by all parties to interpret the information being provided; it will no longer be possible to use a web browser to open your submissions, for example. As with any significant change to the submission standards, there will likely be a number of sponsor organisations looking at their current solution providers to ensure they still have the best technology for their needs.
To achieve the primary goals of the new specification, many updates will be implemented. Let’s have a look at the key changes in eCTD 4.0 here below.
Faster Regulatory Submission Process
- Document re-use.
It is common for the same content to be needed multiple times throughout the lifecycle of an application. Currently, this involves submitting the same document in each sequence that it is required.
With eCTD 4.0 each document will be assigned a Universal Unique Identifier (UUID). This identifier can be referenced in future sequences, avoiding the need to resubmit the content itself.
- Lifecycle enhancements.
To improve the management of content over longer periods of time, it will be possible to replace many documents in the application with one, or to replace one document with many. The legacy ‘append’ operation will be officially eliminated in eCTD 4.0, and it will be possible to rename documents and metadata (e.g. update the trade name).
- Tables of Content.
The hierarchy set out in eCTD v3.2.2 will become a flat structure in eCTD 4.0. Context of Use and Keywords will be used instead to define the content’s location within any eCTD tables of content generated by the viewing tools.
- Study Tagging Files (STFs).
Document Groups will replace the need to use Study Tagging Files, which will no longer be used in eCTD 4.0.
Enhanced Agency and Sponsor Communication
- Two-way communication between sponsor and agency.
The current specification of the eCTD only allows for one-way communication from the sponsor to the agency via individual sequences. All communication from the agency to the sponsor is done separately and does not form a part of the eCTD itself.
eCTD 4.0 will also enable the agency to respond to the sponsor via a sequence, creating a full picture of the entire lifecycle of the application, including any questions or requests for information, in one single place.
- The use of controlled vocabularies.
The increased use of agreed controlled lists is seen as an essential step in ensuring consistent communication between sponsors and agencies. These lists will be controlled by various bodies, including regional authorities, ICH and other third parties. The publishing systems will need to implement these.
Improve global harmonization of the format.
- Standardization of the format with multiple Standards Organizations.
As well as the ICH, eCTD was also developed based on the HL7’s RPS (Regulated Product Submissions) project, with an aim to become an ISO standard. Along with the involvement of Health Authorities and other third parties for controlled vocabularies, the long-term vision of sponsors and agencies is to provide more content from eCTD dossier via structured data (such as that set out in the IDMP standard). This should all lead to improved harmonization of how the eCTD is implemented across different health authorities, where currently there are more differences than envisaged.
- A new XML schema.
This has been designed to support the updated features of the new standard and be more flexible in the long-term.
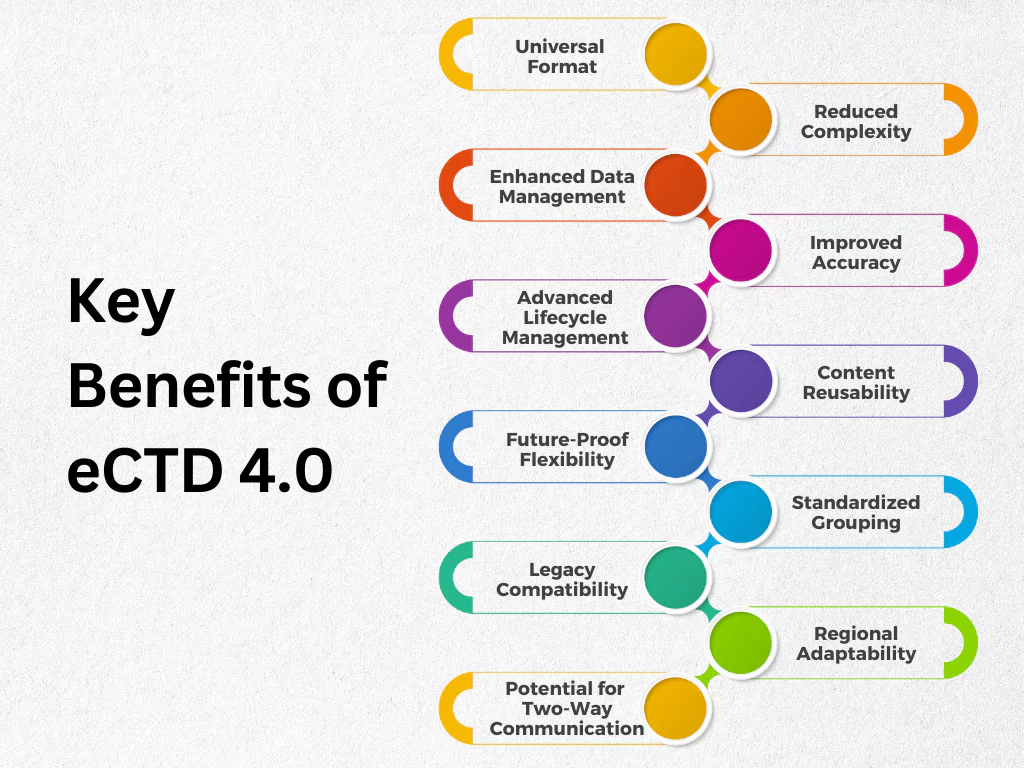
Key Benefits of eCTD 4.0
The current eCTD standard (version 3.x) has limitations that hinder efficiency. The new eCTD 4.0 standard addresses these limitations and offers significant benefits for both regulatory agencies and submitting organizations. eCTD 4.0 aims to improve regulatory submissions. Its key advantages include:
- Universal Format: A single format can be used for submissions to any regulatory agency or region, regardless of the product type (drugs, medical devices, food additives, etc.). This eliminates the need for multiple, region-specific submissions.
- Reduced Complexity: eCTD 4.0 minimizes the need for structural changes and software updates. This streamlines the submission process and reduces overall workload.
- Enhanced Data Management: All content (Modules 1-5) is included in a single, unified exchange message using an XML file. This simplifies data organization and improves consistency.
- Improved Accuracy: Metadata and keyword definitions can be easily corrected without resubmitting entire files. This reduces errors and ensures data integrity.
- Advanced Lifecycle Management: Documents can be replaced, reordered, or their granularity adjusted within the submission while maintaining historical context.
- Content Reusability: Previously submitted documents can be referenced and reused across different submissions and regulatory activities, saving time and effort.
- Future-Proof Flexibility: Controlled vocabularies allow for updates to allowable values without requiring system modifications.
- Standardized Grouping: Documents within sections can be consistently grouped using descriptive titles across all ICH regions.
- Legacy Compatibility: eCTD 4.0 allows for the use and lifecycle management of content created with v3.2.2.
- Regional Adaptability: Additional document metadata can be included to meet specific regional processing needs.
- Potential for Two-Way Communication: The standard lays the groundwork for future two-way information exchange between regulators and submitting entities.
The Impact of eCTD 4.0 on Submission Content Preparation
The PDF format will still be required for much of the content provided in the eCTD. Some minor changes to the ICH guidelines for PDF use have been defined, so it is essential to ensure that the tools used for PDF generation can continue to provide compliant results. The compression method suggested for images has been adjusted for example, so sponsors should ensure they can produce PDFs to the correct new PDF specifications.
Implementing eCTD 4.0
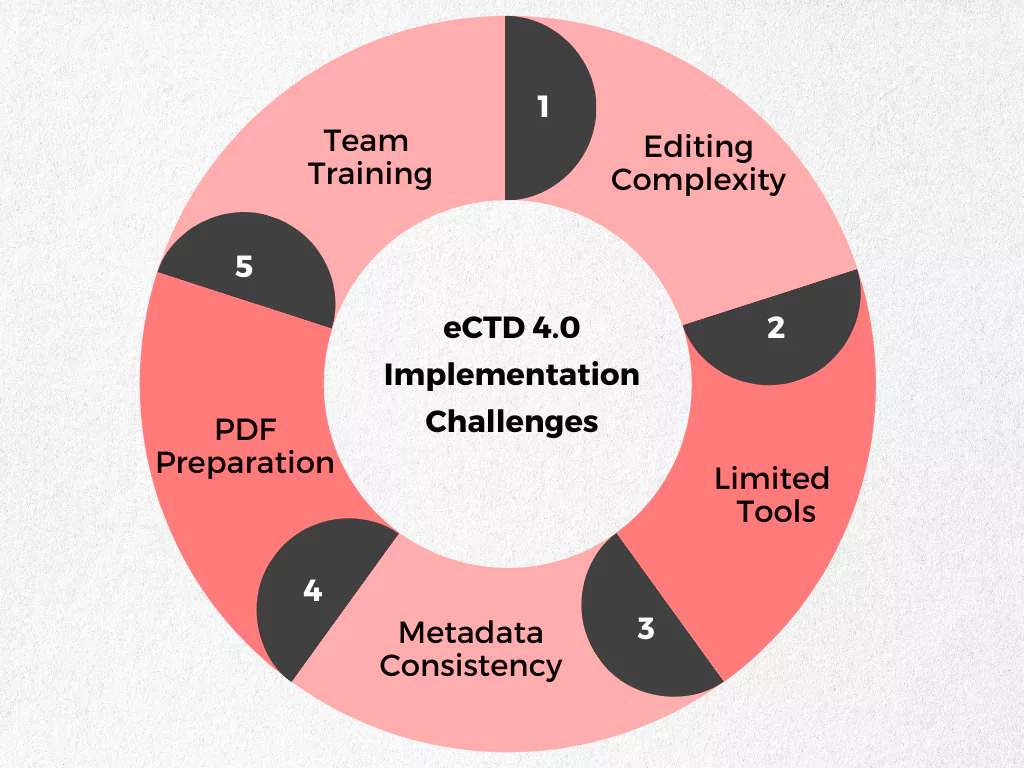
While eCTD 4.0 promises a smoother regulatory submission process, making the switch requires careful planning. Here’s why:
- Complexity: The new format uses complex XML coding, making manual editing difficult. Specialized tools are needed to manage document lifecycles effectively.
- Limited Tools: Currently, there are no easy ways to preview submissions in a web browser.
- PDF Preparation: Existing PDFs may require adjustments to meet the latest compression standards for eCTD 4.0.
- Metadata Consistency: Organizations need to streamline their use of custom terms (metadata) to ensure compatibility with both eCTD 3.2 and 4.0.
- Team Training: Staff require training on the new terminology, concepts, and the latest guidelines from ICH and regional regulatory bodies.
The Overlap Challenge:
Since some regions won’t mandate eCTD 4.0 until 2028, there will be a period where both eCTD 3.x and 4.0 submissions are used. Organizations risk falling behind on regulations if they don’t properly implement eCTD 4.0 alongside maintaining eCTD 3.x support.
For the latest information on eCTD 4.0, please do not forget to regularly check the website of ICH.
Conclusion
eCTD 4.0 represents a significant leap forward in regulatory submissions. By implementing this new standard effectively, organizations can experience a smoother, more efficient submission process with improved data quality and streamlined workflows. Sponsors should embrace these changes, and review their process as a whole, however, and not completely rely on the vendors to provide the total solution for them. It is clear that the publishing vendors in particular will need to make significant changes to their tools, but any software solution requires a well-defined business process to take full advantage of it.
With the introduction of eCTD 4.0 and the first implementations of IDMP, it is clear the next few years will be a busy time for change management within the Life Sciences industry. In the long-run, however, the benefits are clear and will be worth the effort.
Keywords: eCTD v4.0, eCTD 4.0, electronic common technical document 4.0 format, ICH, FDA, regulatory submissions, eCTD version 4.0, eCTD 4.0 implementation, eCTD 4.0 timeline, eCTD 4.0 FDA