eCTD Submission Guidance Hub
This resource provides comprehensive guidance on eCTD submissions. Find comprehensive info on each eCTD submission type by clicking on the information.

NDA Submissions
Discover everything about new drug applications.

ANDA Submissions
Understand how generic drugs reach patients.

IND Submissions
Find answers to for navigating the IND application process in this guide.

BLA Submissions
We break down BLAs so that you can expedite your submission.

510(k) Submissions
Read this guide for a comprehensive understanding of the 510(k) process.

PMA Submissions
Learn how to get FDA clearance for high-risk Class III medical devices.

CTA Submissions
Discover CTA submissions and how to prepare for these.

MAA Submissions
We explore everything you need to know about MAA submissions.
Additional resources on eCTD & Regulatory Submissions
Are you new to eCTD submissions?
Here is some additional info.
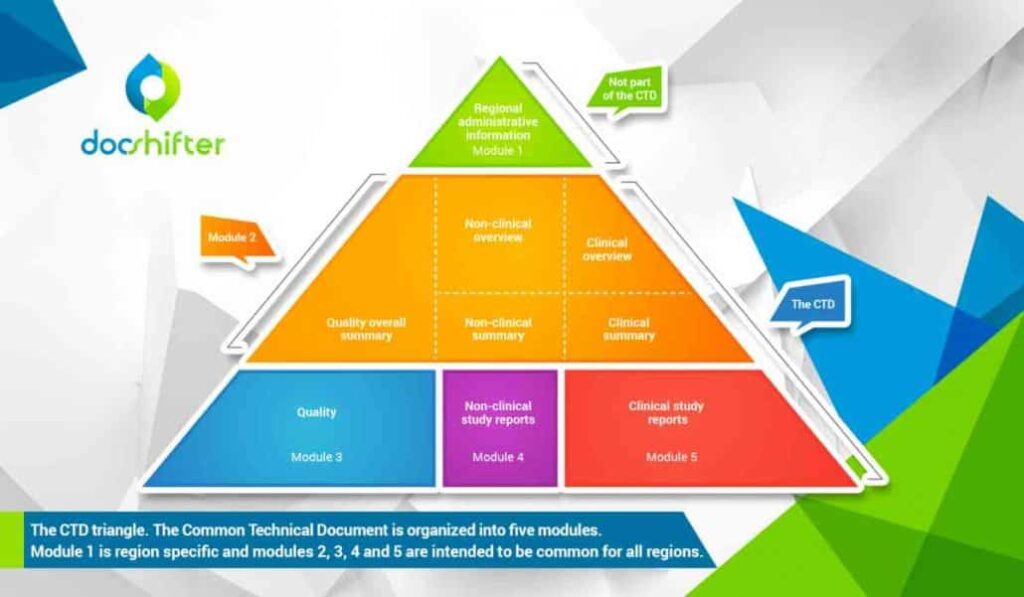
What are eCTD submissions & why are they important?
eCTD is a standardized electronic format for regulatory submissions that has revolutionized the regulatory landscape. By replacing paper-based submissions with electronic files, eCTD offers numerous benefits, including:
- Efficiency: Streamlines the submission process, reducing time and costs.
- Accuracy: Minimizes errors and omissions, ensuring accurate information.
- Global harmonization: Promotes consistency across regulatory agencies.
- Environmental benefits: Reduces paper consumption and waste.
Overall, eCTD has significantly improved the speed, efficiency, and accuracy of regulatory submissions.
Types of eCTD submissions
eCTD submissions can be categorized based on their purpose or content. Here are some common types:
- New Drug Application (NDA): Submissions for new drugs that have not been previously approved.
- Biologics License Application (BLA): Submissions for biological products, such as vaccines and therapeutic proteins.
- Supplemental New Drug Application (sNDA): Submissions to amend or modify an existing NDA or BLA.
- Investigational New Drug (IND): Submissions to conduct clinical trials of a new drug.
- Annual Report: Submissions to provide updates on ongoing clinical trials or marketed products.
- Safety Update Report: Submissions to report adverse events or safety concerns associated with a product.
- Post-Marketing Surveillance Report: Submissions to monitor the safety and efficacy of a product after it has been approved.
- Resubmission: Submissions that have been previously rejected by a regulatory agency and have been revised to address the deficiencies.
These are just a few examples of the various types of eCTD submissions that can be made to regulatory agencies. The specific requirements for each submission type may vary depending on the jurisdiction and the nature of the product being submitted.
Key Components of eCTD Submissions
eCTD submissions are organized into a structured format that consists of several key components:
- Region-Specific Modules (RSMs): These modules contain information specific to a particular regulatory region, such as the United States, Europe, or Japan. They are used to ensure compliance with regional regulatory requirements.
- Data Standards: eCTD uses standardized data formats and structures to ensure consistency and compatibility between submissions. These standards include the Extensible Markup Language (XML) and the Document Type Definition (DTD).
- Metadata: Metadata provides information about the submission, such as the product name, sponsor, and submission type. It helps regulatory agencies efficiently process and review submissions.
- Electronic Common Technical Document (eCTD) Structure: This defines the overall structure of the submission, including the sequence and organization of the various components.
- Electronic Submissions Gateway (ESG): This is a secure electronic platform that allows sponsors to submit eCTD submissions to regulatory agencies. It ensures the integrity and confidentiality of the submissions.
These key components work together to create a standardized and efficient format for regulatory submissions, facilitating the review process and improving regulatory decision-making.
Got questions?
We (real people) are here to answer all your questions.
As long as they are about DocShifter.

Did you enjoy this content?
Join the largest Regulatory Professionals Community on LinkedIn.
Monthly updates from the regulatory landscape.
DocShifter is trusted by companies of all sizes in regulated industries