USE CASE SUBMISSION READY PDF
Submission-ready PDFs for multiple health authorities
Create what the regulators demand: fully searchable, eCTD compliant PDF renditions, without any manual work
Why do companies trust DocShifter
Fully Automated: Set up once and forget
One document to multiple HA compliant PDFs depending on region specific requirements
Reduce time to market
100% Risk Reduction
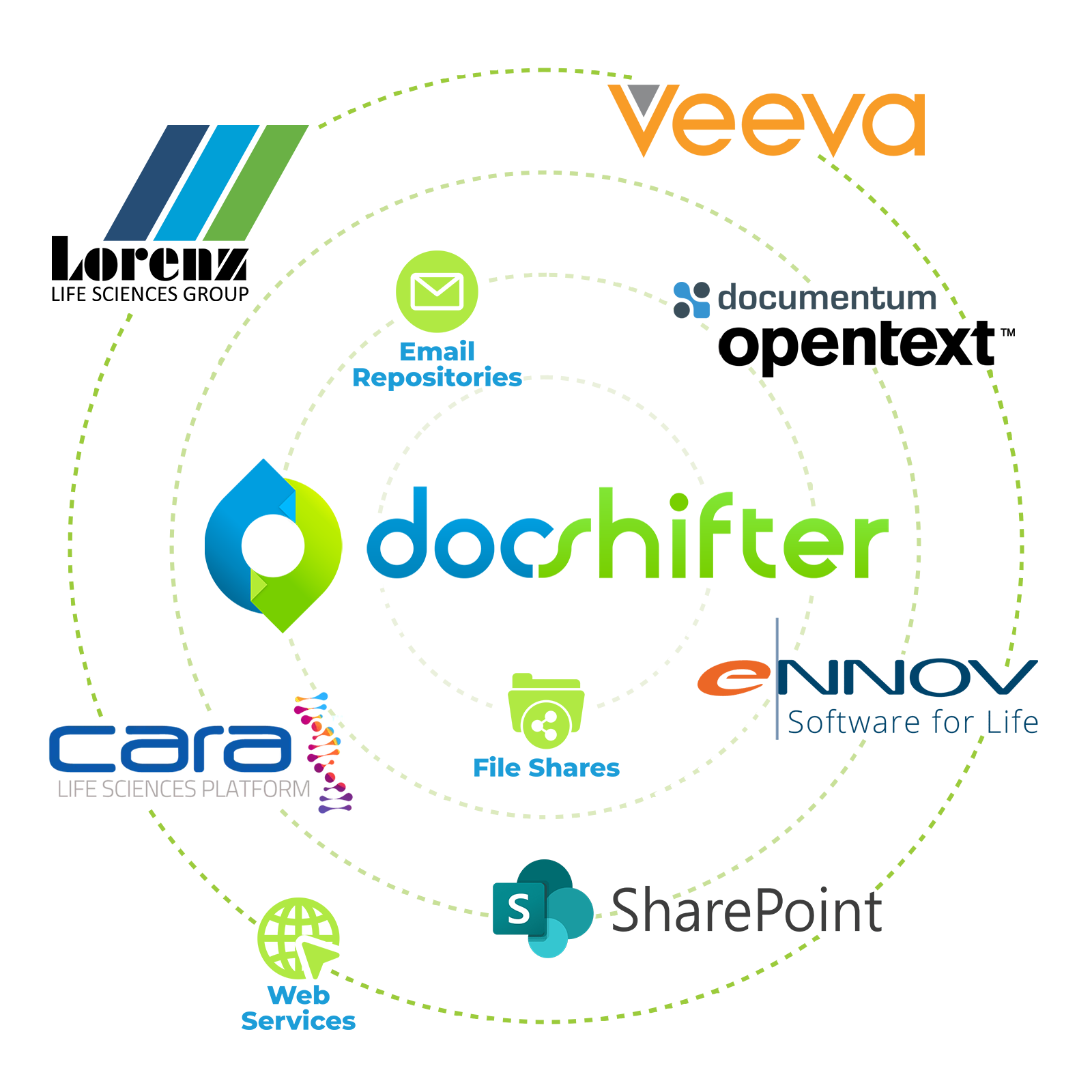
Connect to all your existing systems
Did you know that
that DocShifter was named the most innovative regulatory software provider in 2024 in the Gens & Associates RIM survey?
The Solution
Fully-compliant submission-ready PDFs with DocShifter
DocShifter is a document-to-PDF conversion software with compliance at its core, generating submission-ready renditions for multiple health authorities. Including:
- US FDA
- Health Canada
- MHRA in UK
- PMDA in Japan
- SwissMedic in Switzerland
- CFDA in China
- Thailand
- Australia
- and any other HA
Convert all source files into submission-ready PDFs. Hundreds of configuration options ensure your PDFs meet the most demanding of HA requirements.
Our goal? To facilitate an easy submission. Whether you submit to 1 or more health authorities. Without the human intervention.
Watch our mini demo videos
Mini Demos
Automatically make your PDFs submission-ready.
Thanks to hundreds of PDF configuration options.
Invisible to the Author
- Authors store their documents as usual. A DocShifter workflow will pick up the document automatically and convert to the right format, at the right time
- Workflows automatically route content based on the type of file, metadata, template used, signed or not signed documents, and much more
- Simple to use and highly flexible workflows to define your automation steps
- Check for new documents from multiple content sources (see DS Connectors to find out more)
- Automatically reject conversion tasks with specific characteristics
- Automatically notify people of conversion errors
Flexible Office & Image Handling
- Advanced image handling within documents (dpi, encoding, quality, etc.)
- Advanced image and email format conversions (sizing, orientation, aspect ratio, scaling, and more)
- Advanced Word, Excel, PowerPoint, email, text and image format handling
- Ensure tracked changes convert consistently
- Field code handling to ensure content is up-to-date
- Process or exclude embedded files (including zip, docx, and more)
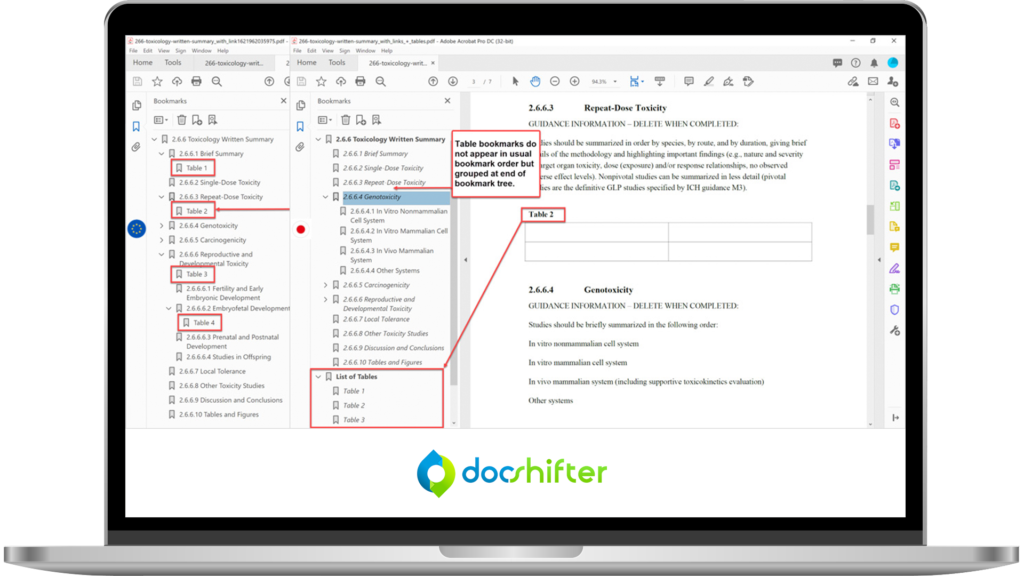
Advanced Bookmark & Hyperlink Handling
- Generate bookmarks from any styles, including your own custom styles
- Ensure hyperlinks are consistently formatted and styled
- Set magnification and zoom level for bookmarks and hyperlinks
- Automatically create bookmarks for PDFs that have no bookmarks (based on text font, size, and more)
Detailed PDF Specification Handling
- Convert from various input formats to PDF or PDF/A
- Select the PDF or PDF/A version of your choice
- Set PDF properties and leverage any DMS metadata
- Flatten PDFs where needed
- Optimize PDF (including for fast web view)
- Ensure PDF viewing preferences are set correctly
Font Handling
- Embed fonts as required (all, subset, nonstandard, and more)
- Automatic font substitutions where fonts are unavailable
- Force specific font substitutions to use available fonts
Advanced Excel to PDF conversion handling
- Embed attachments
- Fit Excel sheet to a PDF page
- Define the Excel paper size for rendering
- Only render selected sheets in an Excel file to PDF
- And many more
Advanced PDF Branding Features
- PDF branding features including watermarks, headers and footers, numbers and many more
Advanced PDF Compression
We can convert multiple file formats into submission-ready PDFs
We can do this from any system

You don't have to worry about these anymore once you have DocShifter
Too many regulations & too little time
Time to market is key for any Life Sciences company. The patients’ health is at stake. Few industries face the same level of regulatory complexity. Tight timelines and different compliance requirements across the globe require strict processes and tools to support them. The right technology is fundamental in ensuring success.
EMA, FDA, PMDA, etc. have specific technical PDF requirements
Each global regulator has similar, but slightly different requirements. While the same content can be reused, the final PDF needs to be different (e.g. bookmark levels set, PDF version, etc.). Automating this PDF preparation process to ensure the right requirements are used is critical. You do not want to do this manually.
Using multiple tools is expensive
From submission content preparation to eCTD submissions, many organizations still rely on multiple, siloed desktop tools (such as MS Office and Adobe technology) – causing high IT infrastructure and licensing costs. By syncing into a single solution, companies can avoid time-to-market delays and stem financial losses.
Manually manipulating PDFs is risky
Using desktop tools to manually handle PDF content is an error-prone task. Embedding fonts, checking hyperlinks, adding ToCs and setting zoom-levels on links and bookmarks to ensure health authority compliance all too often becomes time-consuming. Get it wrong, however, and it can cost a lot more than just your time.
Trusted by life sciences companies of all sizes



















Partnering with confidence for technically compliant, submission-ready PDFs
DocShifter streamlines submissions for a large US-based biotech company by generating 30,000 compliant ready PDFs every month.

A US-based biotech company achieves 60% time savings in document preparaiton and speeds up time to market by 30% thanks to automated PDF checking and fixing.

No more manually merging Microsoft Word files into PDF reports. Automated Report Level Publishing for 510k and PMA Submissions for a Medical Devices Company.


PharmaLex, a technology-enabled solution provider in the Life Sciences industry, partnered with DocShifter to streamline their complex PDF-submission process for their 1000+ clients worldwide. So what changed?

See how we can streamline your submissions
Speak to a specialist for a personalized demo
Frequently Asked Questions
DocShifter can be configured to monitor where you store your submission content: regulatory information management systems, local folders; and based on automated triggers (status change, document update, new document in a monitored location, etc.) it automatically processes the documents.
The way the documents are processed is completely up to the configurations you set. If you are submitting to US FDA, all documents will be rendered to the US FDA PDF specifications; with the correct bookmark settings, inherit zoom options, hyperlinks, table of content at the beginning if your document contains more than 5 pages.
Fully automated.
The same logic applies to any other region you will be submitting to.
Yes. DocShifter can be configured for 'single input' --> multiple output.
DocShifter can render the same source documents in your regulatory information management system to US FDA, HealthCanada, PMDA in Japan specifications. Simultaneously. Results will be slightly different based on the parameters set; and fully compliant for the HA you are submitting to.
In life sciences, common submission requirements for PDFs include adhering to specified PDF versions, ensuring accurate metadata, embedding fonts, structuring for accessibility, validating hyperlinks and bookmarks, implementing security features, following page size and margin guidelines, optimizing image resolution and compression, incorporating functional form fields, supporting digital signatures, and ensuring text searchability. Text searchability is essential for regulatory review, requiring documents to be machine-readable, selectable, copyable, and searchable by text-based queries. Specific requirements vary by regulatory agency, necessitating compliance with agency-specific guidelines such as those provided by the FDA or EMA.
Absolutely. DocShifter seamlessly integrates with every single tool you have in place today, either through native connectors, or through API services.
For more information about connectivity, please visit this page.
It is a standalone applications that easily integrates with multiple other systems such as Veeva, Documentum, Cara. It’s not a plug in as such to these systems. We like to present ourselves as ‘the Switzerland of advanced rendering’, meaning we sit aside, and can easily be coupled with one or more systems in the organisation.
Yes. You can find more information on this on our automated report publishing page.
It integrates with most tools such as Veeva and Cara - where the documents are stored. DocShifter works alongside and connects to all these systems, uses the metadata that is available in those tools.
Based on the DMS you use, there are different triggers available. You can do that completely automated, or use some sort of manual 'I want this document to be rendered right now' process.
In SharePoint, Paul talks about column updates or metadata triggers, but in Veeva, it could be lifecycle update, metadata, or simply monitoring certain document types, for example.
And yes, DocShifter automatically writes the rendered PDF back to your DMS.